Renato Andrade, Hélder Pereira, João Espregueira-Mendes
Asian Journal of Arthroscopy | Volume 1 | Issue 1 | April – Jun 2016 | Page 3-10
Author: Renato Andrade[1],[2],[3], Hélder Pereira[3],[4],[5],[6],[7], João Espregueira-Mendes[2],[3],[5],[6],[8]
[1] Faculty of Sports, University of Porto, Porto, Portugal
[2] Clínica do Dragão, Espregueira-Mendes Sports Centre – FIFA Medical Centre of Excellence, Porto, Portugal
[3] Dom Henrique Research Centre, Porto, Portugal.
[4] Orthopedic Department, Centro Hospitalar Póvoa de Varzim – Vila do Conde, Póvoa de Varzim, Portugal
[5] 3B’s Research Group – Biomaterials, Biodegradables and Biomimetics, Univ. Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and Regenerative Medicine, Avepark – Parque de Ciência e Tecnologia, Zona Industrial da Gandra, 4805-017 Guimarães, Portugal;
[6] ICVS/3B’s–PT Government Associate Laboratory, Braga/Guimarães, Portugal
[7] Ripoll y De Prado Sports Clinic FIFA Medical Centre of Excellence, Murcia-Madrid, Spain
[8] Orthopaedics Department of Minho University, Minho, Portugal
Address of Correspondence
Dr João Espregueira-Mendes
Via Futebol Clube do Porto – F. C. Porto Stadium, Porto, Portugal
Email: espregueira@dhresearchcentre.com
Abstract
The incidence of anterior cruciate ligament (ACL) injuries has been increasing in the last few decades and, along with it, the number of ACL reconstruction failures has also been growing. To overcome the surgical complications and failures, several developments have been made in regard to the ACL treatment. Nowadays, the ACL reconstruction has become more anatomic and individualized, aiming for the closest replication of the native ACL anatomy and biomechanics. As the knowledge regarding the ACL anatomy and biomechanics moves forward, novel surgical techniques and fixation devices have been developed to keep up the patient’s demands and further prevent the early onset of osteoarthritis. Nonetheless, a considerable number of controversies are still under debate. This review will outline the current concepts of ACL treatment, focusing its consensus and controversies.
Key-words: ACL; Anterior cruciate ligament; Reconstruction; Treatment; Current concepts
Introduction
Anterior cruciate ligament (ACL) ruptures are a common injury worldwide, with an estimated incidence of 80,000 to more than 250,000 every year(1), affecting mostly the young athletes under 25 years old. Several risk factors seems to be predisposing the individuals to a higher risk of injury, such as, environmental (meteorological conditions, field surface and footwear), anatomical (Q angle, knee valgus, foot pronation, body mass index, bone morphology – e.g. narrow intercondylar notch, and steeper tibial slopes) and neuromuscular risk factors (altered movement patterns and muscle activation patterns and inadequate muscle stiffness)(1-3). In addition, it was suggested that greater anteroposterior (AP) lengths and height of the lateral femoral condyle in relation to a smaller AP diameter of the lateral tibial plateau can predispose the higher risk of ACL injury (4). If these injuries are left without proper treatment, it will result in increased knee laxity and instability, decreased levels of physical and sporting activities and, eventually lead to degenerative changes of the knee joint(5-8). In this sense, the ACL reconstruction aims to restore the knee stability and function, the normal knee kinematics and prevent the early onset of osteoarthritis.
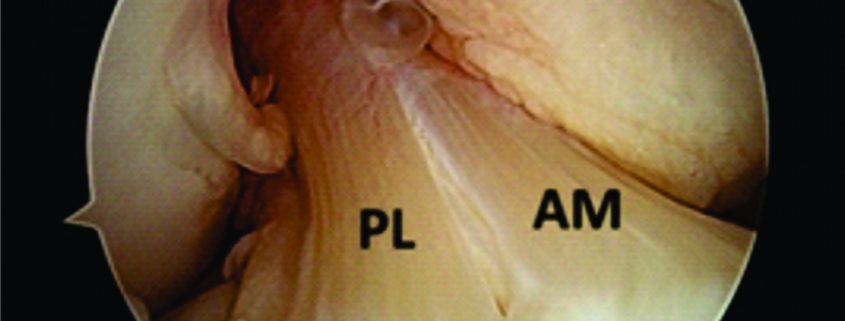
Traditionally, ACL ruptures were surgically treated through non-anatomic ACL reconstructions, with the graft in the isometric position and out of the femoral footprint. However, the non-anatomic ACL reconstruction often resulted residual rotational laxity(9, 10). Nowadays, the focus has shifted towards the anatomic reconstruction highlighting the importance of the correct tunnel position in the native ACL footprint. This concept relies upon the functional restoration of the ACL to its native dimensions, collagen orientation, and insertion sites, taking into account the individual anatomical, morphological characteristics and biomechanical demands of each patient(11). In this sense, Karlsson, Irrgang (12) identified four key principles: restoration of the native insertion site anatomy by placing the tunnels in the correct position; restoration of the two functional bundles (anteromedial and posterolateral; Figure 1); provide the appropriate tension; individualize the surgical procedure for each patient in terms of graft type, tunnel size and graft diameter.
In this review, it will be presented an overview of the current concepts and state-of-art of ACL treatment, focusing the consensus and controversies related to this topic.
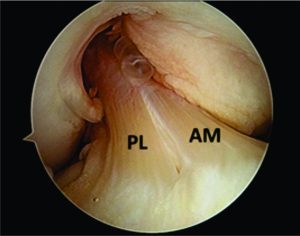
Figure 1: Arthroscopic view of intact native ACL, where it is displayed the two functional bundles, anteromedial (AM) and posterolateral (PL).
Diagnostic procedures and laxity measurement
A comprehensive medical history, musculoskeletal physical examination and imaging procedures play a crucial role in the diagnosis of an ACL injury(13). The taking of medical history should be comprehensive enough to provide information regarding the time and mechanism of injury, rupture pattern and the patient’s activity level(14, 15). The physical examination should comprise valid and reproducible examination tests in order to accurately lead the diagnostic process including the Lachman, pivot-shift and anterior drawer tests. In this sense, the Lachman test is the most sensitive test (87%) and the pivot-shift the most specific (98%)(16). The magnetic resonance imaging (MRI) has been reported to be useful in the ACL injury diagnostics, often capable of identifying complete and partial ACL ruptures (17). In addition, it is helpful in identifying concomitant knee pathology such as other ligament, meniscal, or articular cartilage injury(13). Nonetheless, the abovementioned procedures fail to provide an objective quantitative measure of the ACL laxity. To overcome this issue, several mechanical testing devices have been used in order to measure tibiofemoral AP translation and rotational laxity (18). However, the reliability and diagnostic accuracy of some of them, such as, the KT-1000™, has been questioned(19, 20). Therefore, the ideal tool should be able to assess both “anatomy” and “function” on the same examination. In this sense, the Porto-Knee Testing Device (PKTD) is a safe and MRI-compatible knee laxity testing device, capable of measuring the AP tibial translation and tibial internal and external rotation (Figure 2)(21).
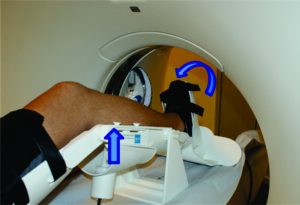
Figure 2: Demonstrative image of PKTD assessment. Left arrow indicates the tibial AP translation induced by the pressure applied in the posterior proximal calf region through the actuators pressurizing. Right arrow indicates the tibial internal rotation through pressure applied at the footplate axis.
Case 1
A twenty-year-old male athlete presented to the sports clinic 4 months following a motorcycle fall. There was no knee effusion evident, however the patient reported residual pain on his right knee. During the physical examination, the patient showed positive Lachman (+/++) and lateral pivot-shift (+) tests, suggesting an increased ACL laxity. Given the medical history and physical examination, the patient was directed for conventional and PKTD MRI examination to assess the presence of further lesions.
The conventional MRI showed bony contusions on the lateral femoral condyle and lateral tibial plateau. In addition, there was evidence of increased signal in one of the bundles, suggesting an ACL partial rupture (Figure 3A). During the PKTD MRI examination, there was tibial PA subluxation (Figure 3B), which increased by 7 mm when submitted to PA stress and internal rotation of the foot (Figure 3C). The conventional MRI examination showed evidence of potential partial rupture, which was confirmed by the PKTD examination, revealing a non-functional ACL.
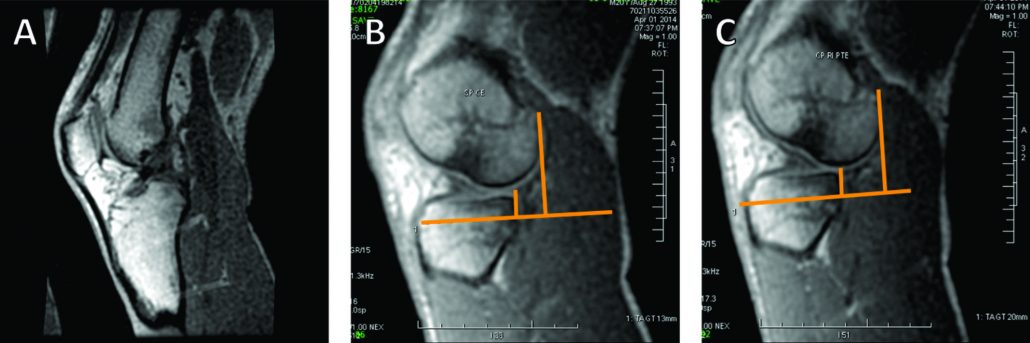
Figure 3: Knee conventional and PKTD MRI examination, with sagittal images of the lateral femoral condyle and lateral tibial plateau of the right knee. A) Knee conventional MRI examination; B) PKTD without stress (13 mm); C) PKTD with PA stress and internal rotation of the foot (20 mm).
In partial ACL ruptures, there is often loss of the functional integrity of the remaining ligamentous fibers, resulting in knee instability and symptomatology of impaired knee function. In cases where the remaining fibers retain their functional capacity, an augmentation procedure will be more suitable
Surgical indications
The decision upon the surgical treatment must be made taking into account the patient’s age, demands of their sports or physical activities, expectation and presence of concomitant injuries(22). In this sense, the indications for ACL reconstruction are young and active adults (18-35 years old) that have sustained an acute ACL injury and signs of instability(13). At this point, concomitant injuries (such as, meniscus, ligamentous or cartilaginous injuries) must be addressed in combination with the ACL reconstruction in order to improve the surgical outcomes(13, 23).
Time-to-surgery
As soon as the decision to operate is made, the surgeon must consider the ideal time to perform the reconstruction. Several prognosis variables (pre-operative range of motion, swelling and quadriceps strength) should be analyzed before proceeding to surgery as these will affect the ACL reconstruction outcome and success(24, 25). Moreover, delaying tACL reconstruction will increase the possibility for the development of other concomitant injuries, such as, cartilage lesions or meniscal lesions(26, 27). In this sense, it has been recommended to perform the ACL reconstruction as soon as pre-operative problems are resolved (no pain, no swelling and at least 90º of flexion)(28) and within 5 months from injury to preserve from further meniscus and/or cartilage damage(13, 29).
ACL reconstruction techniques
The technique for the ACL reconstruction should be taolored to the needs of the patient-tailored and follow the anatomic reconstruction concept. A consensus on which is the best approach, either single-bundle or double-bundle reconstructions, has not been reached however both techniques achieve similar results(13, 30, 31). Thus, the choice of one of these techniques should be based upon different criteria, mostly related to the visualization of the insertion sites and length of tibial and femoral insertion sites (cut-off at 14 mm), which was proposed in ACL reconstruction flowchart by Lesniak et al (11)
Partial ACL ruptures are known to be multifactorial and a consensus on its definition has not been determined (32). In cases which a single bundle (anteromedial or posterolateral) is ruptured or non-functional and the other bundle is well-preserved, a single-bundle augmentation surgery may be deliberated (17, 33, 34). The augmentation technique for the remnant bundle may provide greater vascularization and proprioception, optimize the accuracy of the reconstruction and enhance greater stability and clinical and functional outcomes (33-35). In cases of partial ACL ruptures, the PKTD can be useful in evaluating the individual biomechanical contribution of the remaining bundle functionality (17).
Tunnel placement
Nowadays the anatomic position of the graft within the native footprint has gain increasing popularity and, therefore, the proper tunnel placement plays a crucial role. A 3-portal approach comprising the standard anterolateral and central medial portals and, in addition, an accessory anteromedial portal (superior to the medial joint line approximately 2 cm medial to the medial border of the patellar tendon) has been suggested(36).
Performing the ACL reconstruction with the 3-portal approach will allow the surgeon to visualize the entire ACL and its femoral and tibial insertions(12). In this sense, several landmarks to identify the ACL femoral native footprints have been suggested including lateral intercondylar ridge (most anterior border), lateral bifurcate ridge (division into anteromedial and posterolateral bundles) and the posterior cartilage border (37). If these landmarks are absent, the ACL femoral footprint is known to be at the lower 30-35% of the notch wall with the knee at 90º of flexion. The ACL tibial native footprint can be found through the tibial spines, anterior and posterior horns of the lateral meniscus and posterior cruciate ligament insertion site(12).
Since graft malposition has been reported as one of the most common technical errors(38), several femoral tunnel drilling techniques have been developed, such as, the anteromedial portal, the outside-in and outside-in retrograde drilling techniques(39). It has been shown that transtibial technique yields more subjectively poorly positioned tunnels than the two-incision and medial portal techniques(40). Nevertheless, excellent outcomes have also been reported with a modified transtibial technique(41). In this sense, all the four techniques have shown different advantages and disadvantages, and a clear consensus on which is the best technique for creating the femoral ACL socket has not been reached so far(39). Our recommendations, based on daily practice, is to use the anteromedial and the possibility to add an accessory anteromedial portal.
The accuracy of the tunnel position (tunnel angle and implant position and length) can be further evaluated through radiography, MRI or three-dimensional computed tomography (CT). In this sense, the three-dimensional CT (Figure 4) is considered gold-standard since its measurements provide the highest reliability (42, 43).
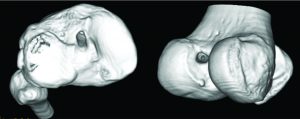
Figure 4: Three-dimensional CT image demonstrating the tibial (image on the left) and femoral (image on the right) tunnel placement in single-bundle ACL reconstruction.
Graft choice
The choice of the correct graft for the ACL reconstruction plays an essential role in the success of the surgery. Regarding the graft choice there is “no one-size-fits-all” concept and, therefore, the decision of the graft should be based on the patient age, size and gender, physical demands, associated injuries, degree of laxity, patient’s anatomy, patient’s choice and expectations and, ultimately, the surgeon preferences, experiences and beliefs. Moreover, the chosen graft should replicate the anatomical and biomechanical properties of the native ligament, guarantee a safe and longstanding fixation, and provide rapid biological integration and low donor-site morbidity(44). In this sense, three different types of graft can be considered including the autografts, allografts and synthetic grafts.
Autografts usually include the bone-patellar tendon-bone (BPTB), the hamstrings tendons (HS) and the quadriceps tendon (QT). The autografts have the advantage of being immediately available and biological healing potential, without risk of additional disease transmission and without additional costs(45). Strong evidence has been shown towards the use of BPTB (Figure 5) or HS grafts once the overall reported follow-up measured outcomes are similar(13). The central QT graft is not recommended for primary ACL reconstruction but often considered for revision cases(45). Recent studies show promising results and low donor-site morbidity levels(46). When comparing the BPTB and HS autografts, the most recent systematic reviews show no significant differences regarding the return to activity, clinical, functional and subjective outcomes (47-50). Nonetheless, the BPBT seems to cause more morbidity (anterior knee and kneeling pain) but increased knee stability, with higher levels of activity(47-50). In addition, other advantages and disadvantages have been pointed out to the different available autografts(15, 22, 44).
The allografts have advantage over the autografts regarding donor-site harvesting morbidity, less operative time and have no limits regarding the number, size and shape(45). Nevertheless, they can result in disease transmission (low risk), higher costs, longer healing time frame and increased risk of failure (specially in young patients and irradiated grafts)(22, 51). The tibialis posterior/anterior, peroneous longus and Achilles tendon allografts are the most commonly used, however the patellar tendon and HS are also easily available(45). Indications for allograft usually included athletes that might be affected by the harvesting symptomatic and functional deficits, ACL revision surgeries and complex multiligament reconstructions(52). When compared to autografts, the current scientific evidence show no significant differences regarding the re-rupture rate, clinical, functional and subjective outcomes(53, 54).
The synthetic grafts are often seen as intra-articular braces and are now into their third generation with several synthetic devices under development. The Ligament Advanced Reinforcement System (LARS) device has shown some favorable outcome in selected patients(55). Their role in ACL reconstruction still remains to be defined(55), however usual indications are rare including healing augmentation in symptomatic and active individuals (>40 years) with an acute ACL injury requiring a fast post-operative recovery(44, 56).
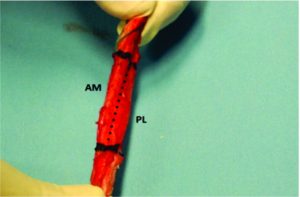
Figure 5: BPBT autograft preparation to single bundle ACL reconstruction. By twisting the autograft 90 degrees, it is possible to approximate the autograft to the native ACL anatomy and biomechanics, resembling the ACL double-bundle anatomy concept (AM, anteromedial bundle; PL, posterolateral bundle).
Graft fixation
Over the last decade, we have been witnessing significant developments concerning the bone plug and soft tissue fixation devices. These fixation devices can be further divided into aperture fixation and suspensory fixation(57). The fixation device for the ACL reconstruction graft should be secure and maintain the optimal tension until full integration of the graft has occurred. In addition, it should provide strength enough to prevent graft failure, stiffness enough to restore stability and provide biomechanical properties to the graft that replicate the native ACL(15, 45). The strength provided should be enough to allow immediate range of movement and weight bearing exercises and permit an early return to sports(57).
The most common bone plug fixation devices for the tibial and femoral fixation are the metal or bio-interferences screws (Figure 6)(15, 45). The bioabsorbable screws have the advantage of faster degradation, promoting the bone ingrowth, incorporation of the graft into the surround tissue, lower need for implant removal and reduced MRI interference(45). Nevertheless, caution should be taken upon the the possible migration of the bioabsorbable screws(58). When considering bioabsorbable against metallic interference screws, both provide similar clinical and functional outcomes, however the bioabsorbable interference screws are more associated with prolonged knee effusion, increased femoral tunnel widening, and increased screw breakage(59).
When considering soft tissue fixation devices, the suspensory devices are more commonly used for the femoral tunnel fixation and the interference screws for the tibial side(15). In regard to the suspensory devices, they have been widely used for graft fixation, providing reduced stiffness than interference screws and higher load to failure. Moreover, it avoids disruption of the insertion site (Figure 7)(15). However, there have been reports of tunnel enlargement(60). When comparing the interference screws with suspensory fixation, corticocancellous fixation and cross biodegradable pins for femoral soft tissue fixation, it was shown that interference screws resulted in decreased risk of surgical failure but no differences were found when postoperative functional outcomes are compared(61). Mechanical properties of cortical suspension and screws fixation for the soft tissue femoral and tibial side are already available in the literature(62, 63).
Biological enhancement of the ACL primary repair
During the past decade, several bio-enhancement tissue engineering regenerative medicine (TERM) approaches have been reported for the primary reconstruction of ACL ruptures, including cell-based therapy, artificial ligament systems, platelet-rich plasma (PRP), growth factors and cytokines, calcium phosphate (hybridized tendon), biodegradable biomaterials and mechanical stimulation (low-intensity pulsed ultrasound). These TERM approaches have been showing promising results as they can work in synergy with the ACL reconstruction and have the potential advantages of enhancing better ligamentization and faster recovery(64). The addition of PRP to ACL treatment has shown promising results in accelerating the graft maturation. However, there is no clear evidence of the benefits of PRP on tunnel and tendon-to-bone healing and enhancing better clinical and functional outcomes(65, 66).
Rehabilitation and prevention
The rehabilitation plays an important role in the success of the ACL reconstruction. In this sense, current trends are towards individualized, patient-tailored, progression-based accelerated (or non-accelerated) rehabilitation protocols in order to achieve better clinical and functional outcomes, as well as, returning faster to the competition. Along this line, the patient adherence and compliance to the rehabilitation protocol are crucial. Moreover, the timeframe of the tissue healing must be respected(67, 68). In addition, these protocols must be adapted to the graft type and concomitant surgical procedures (such as, meniscal or cartilage repair)(69). They include immediately knee full extension, immediate partial weight bearing (in exception when associated lesions are present and a concurrent surgical procedure was performed, such as, meniscus or cartilage repair). Full description of criteria progression-based rehabilitation protocols have already been published in the scientific literature(69, 70).
Prevention programs are the keystone for reducing the rate of non-contact ACL injuries and should focus in adjusting the neuromuscular and biomechanical modifiable risk factors. These often include sportive technique modification, neuromuscular training, stretching, plyometric training, balancing the hamstring/quadriceps ratios, and trunk/core control training(71). A wide range of prevention programs have been developed, with good results being reported(13, 71, 72). In addition, a comprehensive follow-up of the patient’s neuromuscular and biomechanical potential deficits (such as, dynamic knee valgus and high abduction loads) after ACL reconstruction plays a critical role in preventing recurrence of the ACL injury (secondary prevention)(73).
Return to sports
Returning to pre injury level of sports is the main goal of every young athlete but still a controversial issue in the sports medicine community. The timing of returning to competition is multifactorial and therefore several preoperative (age, preoperative rehabilitation, full knee extension and neuromuscular control), intraoperative (graft choice) and postoperative factors (rehabilitation protocol and psychological factors) have been suggested to influence the return to play(74). Clearance to return to competition should be a multidisciplinary decision and take into account objective criteria instead of time frames(67). In this sense, several objective criteria have been proposed and the most important are: no pain or swelling; full active knee range of motion; isokinetic unilateral and bilateral balance and functional hop testing (side-to-side difference <15%); functional and static knee stability(67, 70). In a meta-analysis, comprising a total of 5770 patients (from 48 studies) and a mean follow-up of 41.5 months, 82% of the participants returned to some kind of sports participation, while only 63% returned to their pre-injury level and 44% to competitive sports(75).
Case 2
A 21-years-old amateur male football player presented to the sports clinic reporting symptoms of knee instability (give-away). During the medical history taking, the patient reported that he had 3 years ago an ACL rupture, which was reconstructed with a HS autograft on the 20th day from injury. The surgery and subsequent rehabilitation underwent without any complications and the football player returned to play at the 9th month. During the physical examination, there was present an increased tibial PA and rotation laxity, evidenced by the Lachman (+) and lateral pivot-shift (++) tests, specially when compared to the contralateral healthy knee. There was no knee effusion, stiffness or loss of range of motion. In light of these clinical findings, the patient was referred for MRI with PKTD examination to assess the autograft status and the presence of pathological laxity.
Although the football player underwent all the rehabilitation phases and had returned to competition without complications, three years after the ACL reconstruction he begin to feel symptomatology of instability. The MRI exam with the PKTD showed that he had significant residual laxity on his right knee, with side-to-side differences of 6 mm on the medial side and 10 mm on the lateral side (Figure 8).
Despite the several developments in the orthopaedic surgery, residual laxity after ACL reconstruction is still an issue to overcome. This residual laxity often results from permanent deformation of the graft tissue that precluded the restoration of the normal knee stability. This residual laxity may result from technical errors, such as, graft undertensioning, graft slippage or micromotion (due to improper tibial fixation), incomplete healing (integration of the graft), incorrect tunnel placement, inadequate graft fixation, missed associated laxities (specially, the posterolateral corner laxity) and divergent screws placed (>15º). In addition, traumatic re-rupture, aggressive rehabilitation or early return to play may also lead to residual laxity.
Conclusions
A great deal of focus from the orthopaedic and sports medicine communities has been on the ACL treatment. There is still an open debate in many features of the ACL injury management, while considerable developments have been made over the past few decades. In this review it is outlined and discussed the current consensus and controversies of the ACL treatment and the summary of the key points is presented below.
A complete and reliable diagnostic process should comprise a comprehensive medical history, musculoskeletal physical examination and imaging procedures (radiography and MRI). This can be complemented with laxity measurements with arthrometers (KT-1000) or better with MRI-compatible devices (PKTD).
Young and active adults with acute ACL injury and signs of instability are candidates for ACL reconstruction.
The surgery should be performed after the acute signs are resolved and within the first five months of injury.
Current trends of ACL reconstruction are towards the anatomic and individualized reconstruction.
In partial ACL ruptures, single-bundle augmentation surgery may be an option.
Femoral tunnel placement should be made through a tibial independent approach.
No consensus regarding the graft type (autograft vs. allograft) or autograft source (BPTB vs. HS).
No consensus concerning the graft fixation. For bone plugs fixation, metal or bio-screws are more commonly used. For soft tissue fixation, suspension devices for the femoral side and interference screws for the tibial side.
Although the promising results, the additional value of TERM approaches is not still well established in the literature.
The postoperative rehabilitation should be made through individualized, patient-tailored, progression-based accelerated (or non-accelerated) rehabilitation protocols.
Prevention programs are effective in reducing the rate of non-contact ACL injuries and a comprehensive follow-up of neuromuscular and biomechanical deficits is crucial for the secondary prevention.
The return to competition should be a multidisciplinary decision and be based in objective criteria.
References
1. Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, DeMaio M, et al. Understanding and preventing noncontact anterior cruciate ligament injuries a review of the Hunt Valley II meeting, January 2005 Am J Sports Med. 2006;34(9):1512-32.
2. Andrade R, Vasta S, Sevivas N, Pereira R, Leal A, Papalia R, et al. Notch morphology is a risk factor for ACL injury: a systematic review and meta-analysis. JISAKOS. 2016:jisakos-2015-000030.
3. Pereira H, Fernandes M, Pereira R, Jones H, Vasconcelos J, Oliveira JM, et al. Anterior Cruciate Ligament Injuries Identifiable for Pre-participation Imagiological Analysis: Risk Factors. In: Doral M. N., Karlsson J. editors. Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation. Springer; 2014. p.1525-1536.
4. Pereira H, Fernandes M, Pereira R, Monteiro A, Oliveira J-M, Reis R-L, et al. Radiographic method to determine risk factors for ACL rupture in athletes based in bone morphology. Revue de Chirurgie Orthopédique et Traumatologique. 2013;99(8):e4.
5. Meuffels DE, Favejee M, Vissers M, Heijboer M, Reijman M, Verhaar J. Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med. 2009;43(5):347-51.
6. Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014 Sep;42(9):2242-52.
7. Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014 May;42(5):1049-57.
8. Gomoll A, Filardo G, De Girolamo L, Esprequeira-Mendes J, Marcacci M, Rodkey W, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):450-66.
9. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-83.
10. Georgoulis AD, Ristanis S, Chouliaras V, Moraiti C, Stergiou N. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin Orthop Relat Res. 2007;454:89-94.
11. van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single-and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy. 2010;26(2):258-68.
12. Karlsson J, Irrgang JJ, van Eck CF, Samuelsson K, Mejia HA, Fu FH. Anatomic Single-and Double-Bundle Anterior Cruciate Ligament Reconstruction, Part 2 Clinical Application of Surgical Technique. Am J Sports Med. 2011;39(9):2016-26.
13. Shea KG, Carey JL, Richmond J, Sandmeier R, Pitts RT, Polousky JD, et al. The American Academy of Orthopaedic Surgeons Evidence-Based Guideline on Management of Anterior Cruciate Ligament Injuries. J Bone Joint Surg Am. 2015;97(8):672-4.
14. Geraets SE, Meuffels DE, van Meer BL, Boer HPB, Bierma-Zeinstra SM, Reijman M. Diagnostic value of medical history and physical examination of anterior cruciate ligament injury: comparison between primary care physician and orthopaedic surgeon. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):968-74.
15. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. JISAKOS. 2016:jisakos-2015-000001.
16. Zhang Y, Yao Z, Ma L. Clinical examination of anterior cruciate ligament rupture: a systematic review and meta-analysis. Acta Orthop Traumatol Turc. 2016;50(1):22-31.
17. Ohashi B, Ward J, Araujo P, Kfuri M, Pereira H, Espregueira-Mendes J, et al. Partial Anterior Cruciate Ligament Ruptures: Knee Laxity Measurements and Pivot Shift. In: Doral M. N., Karlsson J. editors. Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation. Springer; 2014. p.1245-1258.
18. Pereira H, Sevivas N, Pereira R, Monteiro A, Oliveira JM, Reis R, et al. New tools for diagnosis, assessment of surgical outcome and follow-up. In: Hernández JA, Monllau JC, editors. Lesiones ligamentosas de la rodilla. Barcelona, Spain: Marge Médica Books 2012. p. 185-98.
19. Forster I, Warren-Smith C, Tew M. Is the KT1000 knee ligament arthrometer reliable? J Bone Joint Surg Br. 1989;71(5):843-7.
20. Graham G, Johnson S, Dent C, Fairclough J. Comparison of clinical tests and the KT1000 in the diagnosis of anterior cruciate ligament rupture. Br J Sports Med. 1991;25(2):96-7.
21. Espregueira-Mendes J, Pereira H, Sevivas N, Passos C, Vasconcelos JC, Monteiro A, et al. Assessment of rotatory laxity in anterior cruciate ligament-deficient knees using magnetic resonance imaging with Porto-knee testing device. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):671-8.
22. Murawski CD, van Eck CF, Irrgang JJ, Tashman S, Fu FH. Operative treatment of primary anterior cruciate ligament rupture in adults. J Bone Joint Surg Am. 2014;96(8):685-94.
23. Lynch TS, Parker RD, Patel RM, Andrish JT, Spindler KP, Amendola A, et al. The Impact of the Multicenter Orthopaedic Outcomes Network (MOON) Research on Anterior Cruciate Ligament Reconstruction and Orthopaedic Practice. J Am Acad Orthop Surg. 2015 Mar;23(3):154-63.
24. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-22.
25. Eitzen I, Holm I, Risberg M. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43(5):371-6.
26. Michalitsis S, Vlychou M, Malizos KN, Thriskos P, Hantes ME. Meniscal and articular cartilage lesions in the anterior cruciate ligament-deficient knee: correlation between time from injury and knee scores. Knee Surg Sports Traumatol Arthrosc. 2015 Jan;23(1):232-9.
27. Newman JT, Carry PM, Terhune EB, Spruiell MD, Heare A, Mayo M, et al. Factors predictive of concomitant injuries among children and adolescents undergoing anterior cruciate ligament surgery. Am J Sports Med. 2015 Feb;43(2):282-8.
28. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-6.
29. Krutsch W, Zellner J, Baumann F, Pfeifer C, Nerlich M, Angele P. Timing of anterior cruciate ligament reconstruction within the first year after trauma and its influence on treatment of cartilage and meniscus pathology. Knee Surg Sports Traumatol Arthrosc. 2015 Oct 16. DOI: 10.1007/s00167-015-3830-2
30. Mascarenhas R, Cvetanovich GL, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, et al. Does double-bundle anterior cruciate ligament reconstruction improve postoperative knee stability compared with single-bundle techniques? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1185-96.
31. Tiamklang T, Sumanont S, Foocharoen T, Laopaiboon M. Double-bundle versus single-bundle reconstruction for an-terior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2012; 11.
32. Colombet P, Dejour D, Panisset J-C, Siebold R. Current concept of partial anterior cruciate ligament ruptures. Orthop Traumatol Surg Res. 2010;96(8):S109-S18.
33. Sonnery-Cottet B, Bazille C, Hulet C, Colombet P, Cucurulo T, Panisset JC, et al. Histological features of the ACL remnant in partial tears. Knee. 2014;21(6):1009-13.
34. Matsushita T, Kuroda R, Nishizawa Y, Araki D, Hoshino Y, Nagai K, et al. Clinical outcomes and biomechanical analysis of posterolateral bundle augmentation in patients with partial anterior cruciate ligament tears. Knee Surg Sports Traumatol Arthrosc. 2015 Jul 11.
35. Siebold R, Fu FH. Assessment and augmentation of symptomatic anteromedial or posterolateral bundle tears of the anterior cruciate ligament. Arthroscopy. 2008;24(11):1289-98.
36. Cohen SB, Fu FH. Three-portal technique for anterior cruciate ligament reconstruction: use of a central medial portal. Arthroscopy. 2007;23(3):325. e1-. e5.
37. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-25.
38. Heard WM, Chahal J, Bach Jr BR. Recognizing and managing complications in ACL reconstruction. Sports Med Arthrosc. 2013;21(2):106-12.
39. Robin BN, Jani SS, Marvil SC, Reid JB, Schillhammer CK, Lubowitz JH. Advantages and disadvantages of transtibial, anteromedial portal, and outside-in femoral tunnel drilling in single-bundle anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1412-7.
40. McConkey MO, Dunn WR, Flanigan DC, Wolf BR. Arthroscopic Agreement Among Surgeons on Anterior Cruciate Ligament Tunnel Placement. Am J Sports Med. 2012;40(12).
41. Haro MS, Riff A, Bach Jr BR. Tips for successful transtibial anterior cruciate ligament reconstruction. J Knee Surg. 2014;27(5):331-42.
42. Meuffels DE, Potters J-W, Koning AH, Brown Jr CH, Verhaar JA, Reijman M. Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta Orthop. 2011;82(6):699-703.
43. Pereira H, Sevivas N, Pereira R, Monteiro A, Sampaio R, Oliveira JM, et al. Systematic Approach from Porto School. In: Siebold R, Dejour D, Zaffagnini S, editors. Anterior Cruciate Ligament Reconstruction. Springer; 2014. p. 367-86.
44. Cerulli G, Placella G, Sebastiani E, Tei MM, Speziali A, Manfreda F. ACL Reconstruction: Choosing the Graft. Joints. 2013;1(1):18.
45. Lord B, Grice J, Cox G, Yasen S, Wilson A. (iii) Anterior cruciate ligament reconstruction–evolution and current concepts. Orthop Trauma. 2015;29(1):12-23.
46. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015 Mar;31(3):541-54.
47. Magnussen RA, Carey JL, Spindler KP. Does autograft choice determine intermediate term outcome of ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2011;19(3):462-72.
48. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-97.
49. Mohtadi N, Chan DS, Dainty KN, Whelan DB. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev.2011;9.
50. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015 Mar;22(2):100-10.
51. Sevivas N, Pereira H, Varanda P, Monteiro A, Espregueira-Mendes J. Revision of Failures After Reconstruction of the Anterior Cruciate Ligament. In: Doral M. N., Karlsson J. editors. Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation. Springer; 2014. p. 463-9.
52. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure a case-control study. Am J Sports Med. 2009;37(12):2362-7.
53. Mascarenhas R, Erickson BJ, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, et al. Is there a higher failure rate of allografts compared with autografts in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(2):364-72.
54. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-20.
55. Batty LM, Norsworthy CJ, Lash NJ, Wasiak J, Richmond AK, Feller JA. Synthetic devices for reconstructive surgery of the cruciate ligaments: A systematic review. Arthroscopy. 2015;31(5):957-68.
56. Shaerf DA, Pastides PS, Sarraf KM, Willis-Owen CA. Anterior cruciate ligament reconstruction best practice: A review of graft choice. World J Orthop. 2014;5(1):23-9.
57. Vaishya R, Agarwal AK, Ingole S, Vijay V. Current Trends in Anterior Cruciate Ligament Reconstruction: A Review. Cureus. 2015;7(11).
58. Pereira HM, Correlo VM, Silva-Correia J, Oliveira JM, Espregueira-Mendes J. Migration of “bioabsorbable” screws in ACL repair. How much do we know? A systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):986-94.
59. Mascarenhas R, Saltzman BM, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, et al. Bioabsorbable versus metallic interference screws in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(3):561-8.
60. Baumfeld JA, Diduch DR, Rubino LJ, Hart JA, Miller MD, Barr MS, et al. Tunnel widening following anterior cruciate ligament reconstruction using hamstring autograft: a comparison between double cross-pin and suspensory graft fixation. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1108-13.
61. Colvin A, Sharma C, Parides M, Glashow J. What is the best femoral fixation of hamstring autografts in anterior cruciate ligament reconstruction?: a meta-analysis. Clin Orthop Relat Res. 2011;469(4):1075-81.
62. Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43(1):154-60.
63. Aga C, Rasmussen MT, Smith SD, Jansson KS, LaPrade RF, Engebretsen L, et al. Biomechanical comparison of interference screws and combination screw and sheath devices for soft tissue anterior cruciate ligament reconstruction on the tibial side. Am J Sports Med. 2013;41(4):841-8.
64. Hogan MV, Kawakami Y, Murawski CD, Fu FH. Tissue engineering of ligaments for reconstructive surgery. Arthroscopy. 2015;31(5):971-9.
65. Figueroa D, Figueroa F, Calvo R, Vaisman A, Ahumada X, Arellano S. Platelet-rich plasma use in anterior cruciate ligament surgery: systematic review of the literature. Arthroscopy. 2015;31(5):981-8.
66. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? K nee Surg Sports Traumatol Arthrosc. 2009;17(6):676-82.
67. Espregueira-Mendes J, Pereira R, Monteiro A, Pereira H, Sevivas N, Varanda P. Sports and anterior cruciate lesions. Revue de Chirurgie Orthopédique et Traumatologique. 2011;97(8):S472-S6.
68. Espregueira-Mendes J, Pereira RB, Monteiro A. Lower limb rehabilitation. In: Margheritini F, Rossi R, editors. Orthopedic Sports Medicine: Springer; 2011. p. 485-95.
69. Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601-14.
70. Van Grinsven S, Van Cingel R, Holla C, Van Loon C. Evidence-based rehabilitation following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1128-44.
71. Alentorn-Geli E, Myer GD, Silvers HJ, Samitier G, Romero D, Lázaro-Haro C, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):859-79.
72. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-55.
73. Hewett TE, Myer GD, Ford KR, Heidt RS, Colosimo AJ, McLean SG, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes a prospective study. Am J Sports Med. 2005;33(4):492-501.
74. Ellman MB, Sherman SL, Forsythe B, LaPrade RF, Cole BJ, Bach Jr BR. Return to play following anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2015;23(5):283-96.
75. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011 Jun;45(7):596-606.
| How to Cite this article:. Andrade R, Pereira H, Mendes JE. ACL Treatment in 2016 – Controversy and Consensus. Asian Journal of Arthroscopy Apr- June 2016;1(1):3-10 . |
 Dr. Renato Andrade |
 Dr. Hélder Pereira |
 Prof. Dr. João Espregueira-Mendes |