Meniscal Scaffolds in the Clinics: Present and Future Trends
Ricardo Bastos, Renato Andrade, Hélder Pereira, J Miguel Oliveira, Rui L Reis, Scott Rodeo, João Espregueira-Mendes.
Volume 1 | Issue 2 | Aug – Nov 2016 | Page 47-52.
Author: Ricardo Bastos[1,2,3], Renato Andrade[2,3,4], Hélder Pereira[5,6,7,8], J Miguel Oliveira, Rui L Reis, Scott Rodeo, João Espregueira-Mendes[2,3,5,6,14].
[1] Universidade Federal Fluminense, Nireói, Rio de Janeiro, Brazil.
[2] Clínica do Dragão, Espregueira-Mendes Sports Centre – FIFA Medical Centre of Excellence, Porto, Portugal.
[3] Dom Henrique Research Centre, Porto, Portugal.
[4] Faculty of Sports, University of Porto, Porto, Portugal.
[5] 3B’s Research Group – Biomaterials, Biodegradables and Biomimetics, University of Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and Regenerative Medicine, AvePark- Parque de Ciência e Tecnologia, 4805-017 Barco, Guimarães, Portugal.
[6] – ICVS/3B’s – PT Government Associated Laboratory, Braga/Guimarães, Portugal.
[7] – Orthopaedic Department, Centro Hospitalar Póvoa de Varzim – Vila do Conde, Póvoa de Varzim, Portugal.
[8] – Ripoll y De Prado Sports Clinic FIFA Medical Centre of Excellence, Murcia-Madrid, Spain.
[9] – Co-Chief Emeritus, Sports Medicine and Shoulder Service, Hospital for Special Surgery, New York, USA.
[10] – Co-Director, Tissue Engineering, Regeneration, and Repair Program, New York, USA.
[11] – Orthopaedic Surgery, Weill Medical College of Cornell University, New York, USA.
[12] – Attending Orthopaedic Surgeon, Hospital for Special Surgery, New York, USA.
[13] – Head Team Physician, New York Giants Football, New York, USA.
[14] – Orthopaedics Department of Minho University, Minho, Portugal.
Address of Correspondence
Dr. João Espregueira-Mendes; Via Futebol Clube do Porto – F. C. Porto Stadium, Porto, Portugal; +351 220 100 100; Email: espregueira@dhresearchcentre.com
Abstract
Despite the high incidence, meniscal lesions still remain a clinical challenge due to its limited regenerative ability. In the last two decades, the development of scaffolding strategies has revolutionized meniscus treatment possibilities. Along with these new developments, the orthopaedic community has embraced the campaign “preserve the meniscus”. In this sense, acellular or cellularized scaffolds have emerged as a potential solution to treat irreparable meniscal lesions. Herein, it are overviewed the up-to-date acellular meniscal scaffolds used in the clinics, indications and discussed their outcomes.
Keywords: Meniscal scaffolds; Meniscal implants; Meniscal substitutes.
Introduction
The menisci have been described as a two-edge shaped semilunar discs of fibrocartilaginous tissue, found at the medial and lateral compartment of the tibiofemoral joint (1, 2). They play a fundamental role in many aspects of knee function, including articular congruency and stability, load distribution, shock absorption as well as a role in joint lubrication and proprioception (3). Many of these functions are achieved through the ability to transmit and distribute load over t The medial anhe tibial plateaus.d lateral menisci can transmit from 50% up to 70% of the load when the knee is in extension, and up to 85% at 90 degrees of knee flexion (4). Removal of the medial meniscus can result in a 50% to 70% reduction in femoral condyle cartilage contact area and a 100% increase in contact stress (5). Total lateral meniscectomy causes a 40% to 50% decrease in cartilage contact area and increases contact stress in the lateral compartment up to 200% to 300% of normal. Furthermore, even just partial removal of the meniscus does alter joint loading, particularly when two thirds of the posterior horn is excised (6). Despite the importance of the meniscus structure and the need for its preservation, meniscal lesions are the most common surgically treated knee pathology, and their annual incidence can be estimated at 60-70 per 100,000 knees, with 850,000 meniscal procedures performed yearly only in the United States (7) and 400,000 in Europe (8). For several years, the meniscus function was not fully understood. Recent pre-clinical and clinical evidences support the idea that the preservation of the meniscus structure is of outmost importance (9, 10). Thus, tissue engineering approaches have gain great attention as promise to regenerate different tissues and organs, including meniscus tissue (11-15). It has provided a fundamental understanding and technology that have permitted the development of scaffolds derived from biological tissues and synthetic materials, and there is currently a large amount of active, ongoing research into meniscus scaffolds (16-18). The meniscus scaffolds have been mainly limited to the treatment of meniscus partial repair once it requires an undamaged meniscal rim and enough tissue at the anterior and posterior horns to allow the fixation of the scaffold to the remaining meniscal tissues.
Types of Scaffolds
Scaffold biomechanical structure must have adequate material properties to allow tissue regeneration, while protecting the newly-forming tissue from excessive stresses. Their absorption must be sufficiently gradual, allowing appropriate cell migration, formation of new vessels, and matrix synthesis in order to create meniscal-like tissue (19, 20). At the same time, the scaffold and its degradation products should not damage the articular surface or invoke a foreign body reaction. An important step in the preparation of acellular meniscal scaffolds is the ability of mimicking the architectural and geometric complexity of the native tissue (20, 21). In this sense, it is crucial to further understand the menisci anatomy, biology, ultrastructure and biomechanical function to enhance the success of the meniscal substitution (1, 13). Two scaffolds are currently in clinical use.
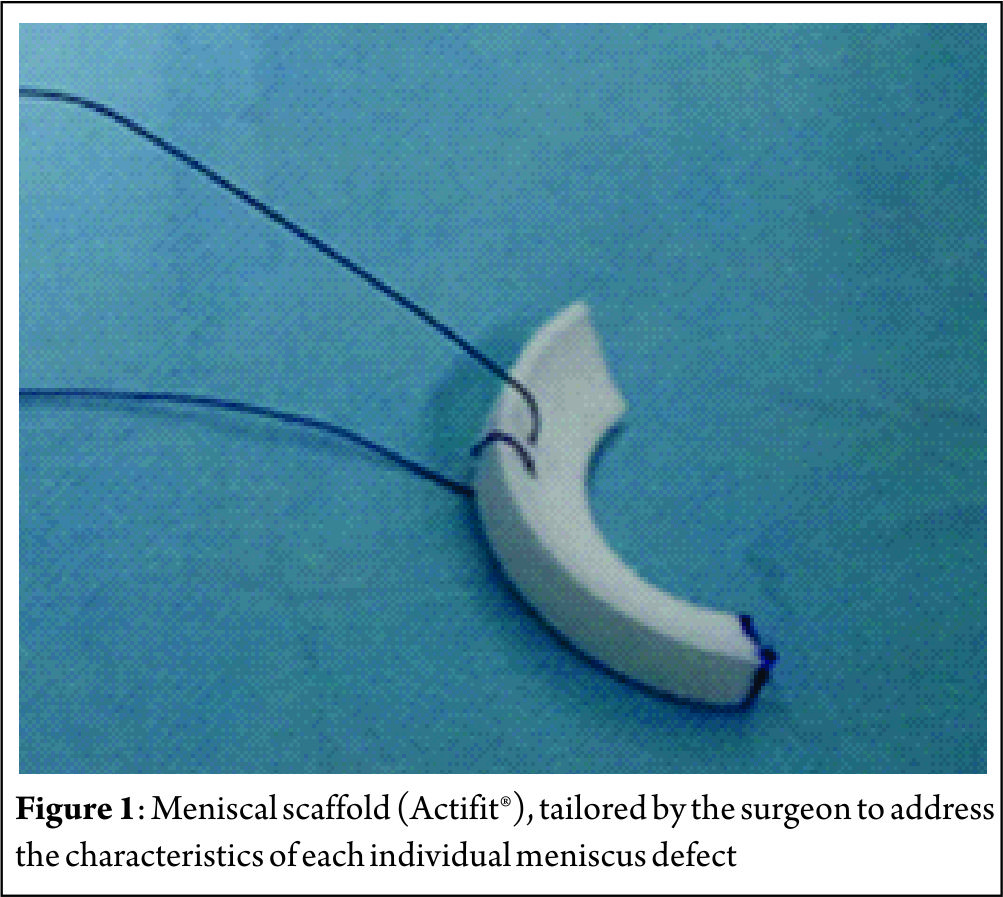
Collagen Meniscus Implant (CMI, Ivy Sports Medicine GmbH, Germany) – First published in 1997, CMI is a type-I collagen (isolated and purified from bovine Achilles tendon) scaffold (22) to which glycosaminoglycans are added. It has a meniscus-like shape, is implantable arthroscopically, and it is biocompatible and biodegradable. It has a microscopic porous structure that allows cellular ingrowth, induces differentiation and proliferation of fibrocartilaginous cells, leading to the creation of a meniscus-like tissue, concomitant with gradual resorption of the scaffold. Nevertheless, collagen scaffolds are fragile during the implant procedure, and have shown a decrease in size on follow-up magnetic resonance image (MRI) and arthroscopic second look follow-up. The second type of scaffold is Actifit® (Orteq, United Kingdom) that has been developed to overcome the perceived limitations of CMI related to difficulties in tissue handling with respect to suturing during implantation (Figure 1). Actifit® is composed of a slowly degrading polymer with polycaprolactone and urethane segments (23). Its structure seems to have better mechanical properties and is more resistant to sutures and loads as compared to CMI. The scaffold is 80% porous; the remaining 20% are made of a polymer with a low absorption rate. Degradation starts with hydrolysis of polycaprolactone segments, which lasts up to five years; the polyurethane segments are removed by macrophages and giants cells or integrated into surrounding tissues (24, 25).
Indications – Contraindications
When considering meniscal scaffolding, the surgeon should take into account several individual aspects, such as the patient’s age and weight, status of meniscal degeneration or concomitant conditions (such as axial malalignment and ligamentous insufficiency) (26). In this sense, several indications and contraindications have been developed as summarized in Table 1.
Preoperative Preparation
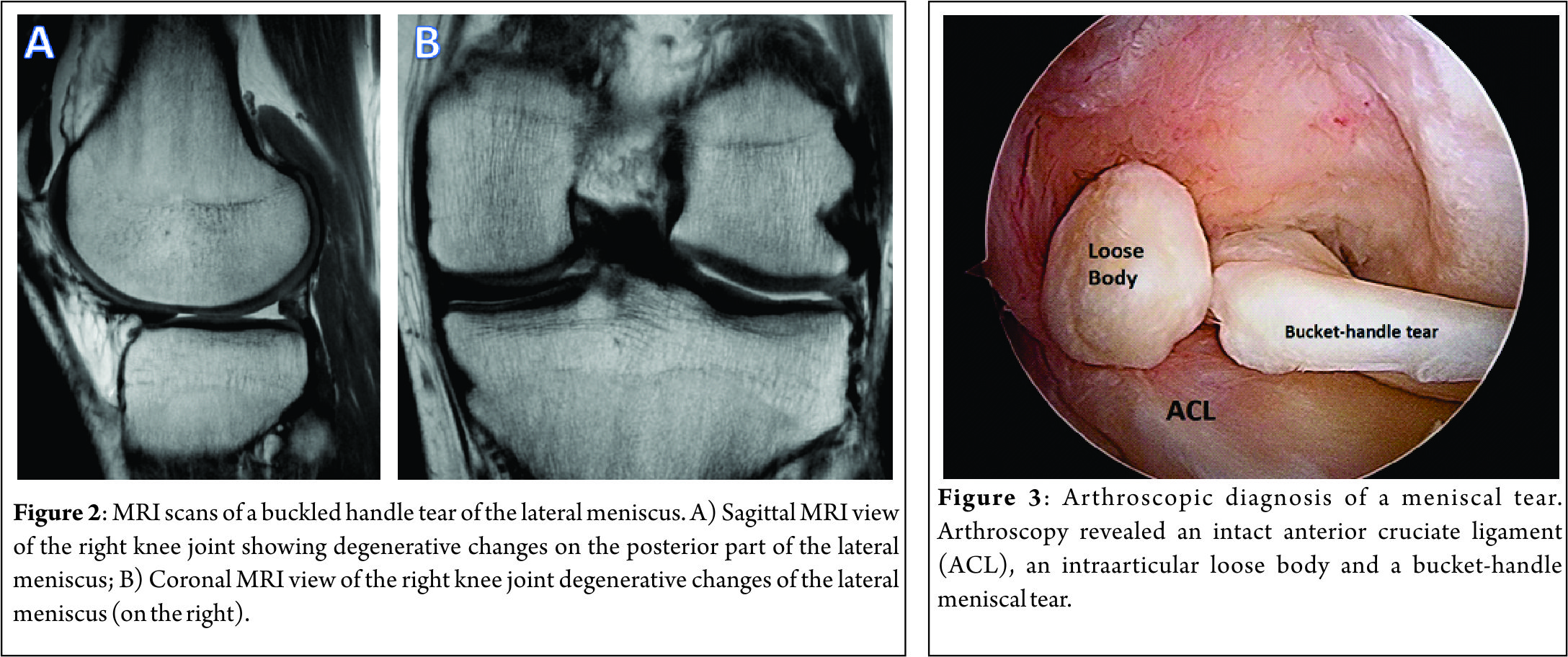
The preoperative imaging preparation usually involves radiography, MRI and, in some special cases, an arthro-computed tomography (arthro-CT). The radiographic imaging studies usually include bilateral comparison of weight-bearing radiographs (antero-posterior, lateral, Schuss or Rosenberg views). The MRI is usually performed to assess the cartilaginous structures status, quantify the meniscal damage, as well as the presence of bone marrow edema and/or meniscal extrusion (Figure 2). The arthro-CT scan may complement the MRI studies by assessing the meniscal volume and chondral damage (26). The imaging studies should be complemented with a comprehensive clinical examination of the knee. Special attention should be given to the knee ligament stability, as this has several implications in the meniscal surgery. In addition, diagnostic arthroscopy (Figure 3) may be performed to further assess the meniscal status and decide upon the best technique (26).
Surgical Technique
The procedure can be performed arthroscopically using the two standard anteromedial and anterolateral portals. The portals should be enlarged for an easier passage of the scaffold. The native remaining meniscus is thoroughly evaluated, and any torn or degenerative tissue is removed in order to leave a healthy and uniform meniscal rim, ensuring that the resulting defect site extends into the vascularized red-on-red or red-on-white zone of the meniscus. The meniscal rim is punctured in order to create vascular access channels. Gentle rasping of the synovial lining may further stimulate meniscal integration and tissue ingrowth. The exact size of the defect is measure with a flexible rod loaded in a rigid cannula starting at the posterior end of the lesion. The scaffold is measured and trimmed to the correct size on the sterile field of the operating environment (10% larger than in situ measurement to compensate for the shrinkage caused by suturing of the sponge-like material and to assure a snug optimal fit into the prepared defect). In order to achieve a perfect fit of the scaffold with the native meniscus at the anterior junction, the anterior side should be cut at an oblique angle of 30°-45°. The implant is inserted into the defect (Figure 4). Standard arthroscopic meniscal suturing techniques may be utilized for scaffold stabilization. The authors prefer “all-inside” vertical stitches placed every 4 to 5 mm to suture the scaffold along the periphery. The anterior and posterior scaffold extremities are fixed to the native remnant with horizontal stitches.
Concomitant Surgeries
Since other associated deficiencies (such as axial malalignment or ligamentous instability) may lead to poorer outcomes following meniscal surgery, these should be address in combination with the meniscal substitution (27). Anterior cruciate ligament insufficiency, if not addressed, may result in residual laxity, which may lead to an unfavorable meniscal healing environment. In this sense, ACL reconstruction has been performed along with the meniscal substitution in up to 67% of the patients (28-30). When performing concomitant ACL reconstruction, the meniscal bed should be firstly prepared and then the tibial and femoral tunnels may be drilled. After the tunnels are drilled, the ACL graft is passed through the tunnels and fixed at the femoral site, as the meniscal scaffold is inserted and sutured. Subsequently, the ACL graft is fixed at the tibial site with 20° of knee flexion (31). When uncorrected axial knee malalignments are found, these should be concomitantly or previously corrected. In a varus malalignment situation, a high tibial osteotomy may be performed to correct the malalignment. Special attention must be directed to the tibial slope and proper release of the medial collateral ligament should be performed. In valgus malalignments, if the deformity does not involve the tibial bone, osteotomy is done on the femoral side to avoid joint line obliquity (27).
Rehabilitation protocol
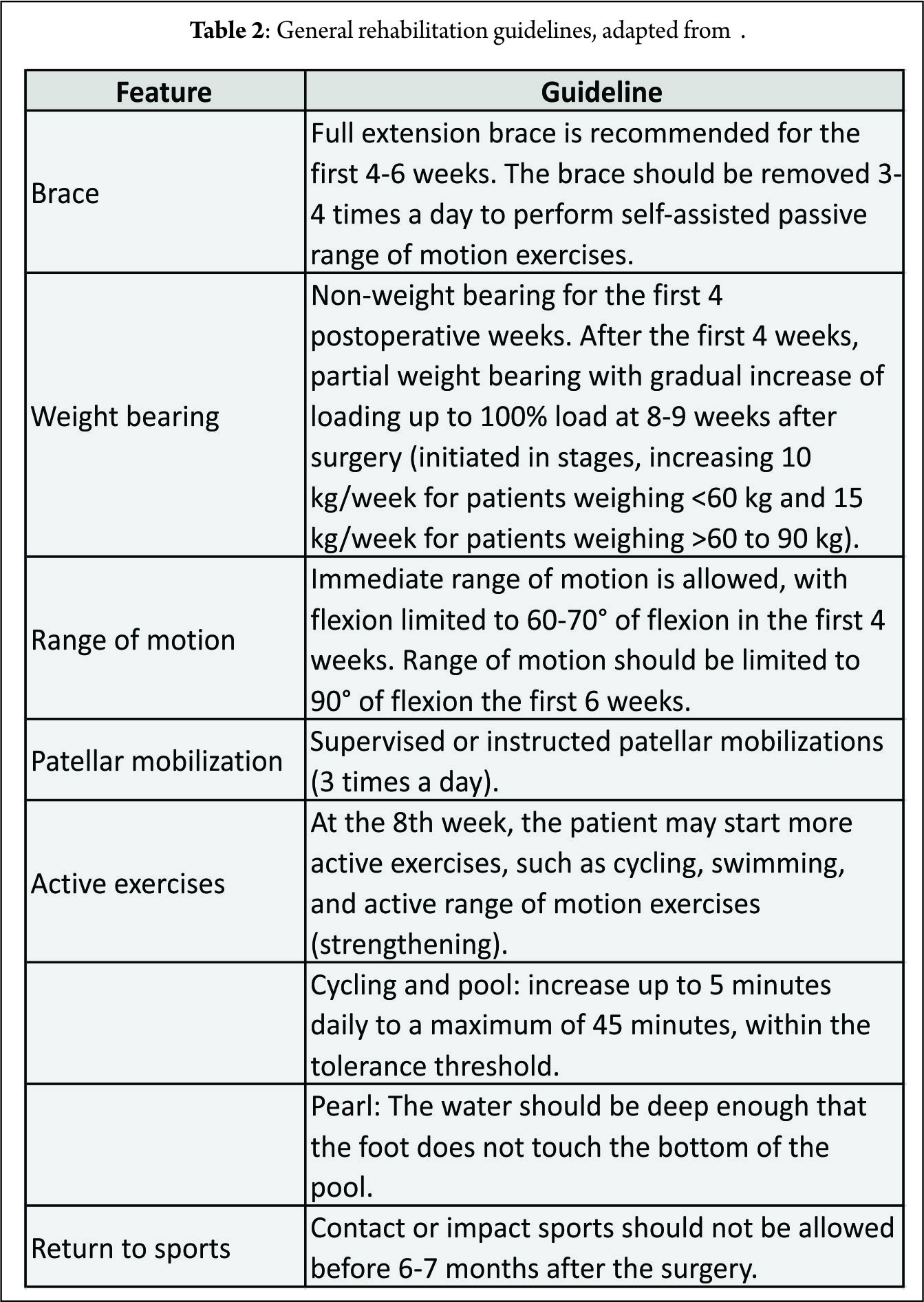
Patients are required to undergo a conservative rehabilitation program similar to that for a meniscal allograft. Special attention is required when the meniscal scaffold is implanted with concomitant ACL reconstruction or realignment osteotomy. In these cases, a rehabilitation program should be tailored to comply with the concomitant procedures postoperative particularities (26, 27). General guidelines for the rehabilitation program are presented in Table 3.
Clinical Studies
Although the literature contains clinical studies (33-35) that support the use of meniscal scaffold implantation for the treatment of irreparable meniscal tears, the quality of the studies is generally low, with lack of randomized trials and long-term follow-up to confirm clinical benefit and the most appropriate indications. Furthermore, long-term follow-up studies are required to verify the protective effect on the damaged joint compartment exerted by meniscal scaffold implantation.
A recent systematic literature review (35) analyzed results and indications for the treatment of meniscal loss. There has been an increase in publications regarding this topic recently, and the authors concluded that both CMI and Actifit seem to be safe and positive results have been shown for both scaffolds. Bulgheroni et al. (36) evaluated the safety and effectiveness of the polyurethane meniscal scaffold through clinical examination, MRI and arthroscopic second look, over a minimum two-year follow-up and showed no adverse reactions to the implant. The implant showed clear, hyperintense signal, sometimes irregular, and the chondral surface was preserved in all cases. At arthroscopic second look at 12 and 24 months, the scaffold was found to have an irregular morphology and to be slightly reduced in size. Zafagnini et al. (37), in a 10-year follow-up study, compared the medial collagen meniscus implant versus partial medial meniscectomy. The CMI group showed significantly lower visual analog scale scores for pain and higher objective International Knee Documentation Committee and Tegner index scores. Radiographic evaluation showed significantly less medial joint space narrowing in the CMI group compared to partial medial meniscectomy. No significant differences between groups were reported regarding Lysholm and Yulish scores. Another long-term study compared outcomes of CMI versus partial meniscectomy in patients with concomitant ACL reconstruction. The authors concluded that patients with chronic meniscal tears treated with medial CMI reported lower levels of post-operative pain compared to meniscectomy, while acute lesions treated with CMI showed less knee laxity at follow-up (38). The CMI when performed in the acute setting showed no additional benefits when compared to partial medial meniscectomy alone (28).
Zafagnini et al. (39), in a multi-center study, evaluated the clinical outcomes of 43 patients after lateral CMI implantation. They reported improvement of all clinical scores from baseline to follow-up evaluations. At the final follow-up, 58% of the patients reported activity levels comparable to their pre-injury values, with 95% patient reported satisfaction. A higher body mass index, the presence of concomitant procedures, and a chronic injury pattern were identified as potential negative prognostic factors.
As far as concomitant open-wedge high tibial osteotomies is concerned, Gelber et al. (40) found no short-term additional benefit when compared to partial meniscectomy and meniscal scaffolding.
Final Remarks and Future Directions
The menisci are known to be heterogeneous complex structures with segmental variations according to their anatomy, biology and function. The proper understanding on the different types of meniscal injuries (both traumatic and degenerative) and their pathophysiology and pathomechanics will assist the clinician in identifying the correct indications and contraindication for each type of lesion, preserving the meniscus whenever possible. The clinical application of meniscal scaffolds is limited to CMI and Actifit. In order to successfully implant these meniscus scaffolds, it is required an intact meniscal rim and sufficient meniscal tissue at the anterior and posterior meniscus horns to attach the scaffold. When in case of axial malalignments and/or ligament insufficiencies, these must be correct prior or during the scaffold implantation. The rehabilitation protocol should be tailored to address each patient’s individual characteristics, respect the chronobiology of the scaffold tissue integration and the progression within phases should be goal-based. Novel meniscal scaffolds have been developed for addressing total meniscus reconstruction with a functional meniscus replacement, mimicking the biology and mechanical properties of the native meniscus. These novel scaffolds may further protect the articular cartilage surface of the knee joint from the extensive damage after a total meniscectomy. A second generation of implants pre-cultured in vitro allows cell adhesion and extracellular matrix production and then are implanted into the meniscal defects which will probably follow as cell seeding as has been demonstrated to improve the mechanical properties and histological results. In the future, it may be possible to improve tissue formation in the meniscal scaffold using autologous cells (e.g., stem cells) and/or growth factors (e.g., platelet-rich plasma). This strategy may augment the tissue regeneration and improve clinical results. The use of mesenchymal stem cells may also enhance a greater promotion of intrinsic meniscal healing capacity. In addition, nanotechnology and gene therapy have emerged as potential options and have showed great potential for the treatment of meniscal lesions, however its translation into the clinical setting may take a few more years. Biofabrication of patient-specific meniscal scaffolds with a 3D printer from the advanced segmentation of menisci knee MRI datasets has been showing promising results in the laboratory setting. This novel technique will allow tailoring the meniscal scaffold to the patient-specific native characteristics of the knee.
References
1. Pereira H, Cengiz IF, Silva-Correia J, Cucciarini M, Gelber PE, Espregueira-Mendes J, Oliveira JM, Reis, RL. Histology-Ultrastructure-Biology. In: Hulet C, Pereira H, Peretti G, Denti, M, editors. Surgery of the Meniscus. Springer; 2016. p. 23-33.
2. Halewood C, Amis AA. Physiology: Biomechanics. In: Hulet C, Pereira H, Peretti G, Denti, M, editors. Surgery of the Meniscus. Springer; 2016. p. 35-45.
3. Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J, van Dijk CN. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):423-35.
4. Ahmed A, Burke D. In-vitro of measurement of static pressure distribution in synovial joints—Part I: Tibial surface of the knee. J Biomech Eng. 1983;105(3):216-25.
5. Watanabe Y, Van Scyoc A, Tsuda E, Debski RE, Woo SL. Biomechanical function of the posterior horn of the medial meniscus: a human cadaveric study. J Orthop Sci. 2004;9(3):280-4.
6. Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomech Eng. 1983;5(2):157-61.
7. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013:0363546513495641.
8. Verdonk PC, Dhollander AA, Tampere T, Verdonk R. Meniscus Substitution: Scaffolds, Allografts and Prosthetic Implants. In: Emans PJ, Peterson L, editors. Developing Insights in Cartilage Repair. Springer; 2014. p. 253-65.
9. Verdonk R. The meniscus: past, present and future. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):145-6.
10. Di Matteo B, Tarabella V, Filardo G, Viganò A, Tomba P, Marcacci M. Thomas Annandale: the first meniscus repair. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):1963-6.
11. Pereira H, Frias AM, Oliveira JM, Espregueira-Mendes J, Reis RL. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 2011;27(12):1706-19.
12. Cengiz IF, Pereira H, Pêgo JM, Sousa N, Espregueira‐Mendes J, Oliveira JM, Reis RL. Segmental and regional quantification of 3D cellular density of human meniscus from osteoarthritic knee. J Tissue Eng Regen Med. 2015. doi: 10.1002/term.2082.
13. Pereira H, Caridade S, Frias A, Silva-Correia J, Pereira D, Cengiz I, Mano JF, Oliveira JM, Espregueira-Mendes, J, Reis, RL. Biomechanical and cellular segmental characterization of human meniscus: building the basis for Tissue Engineering therapies. Osteoarthritis Cartilage. 2014;22(9):1271-81.
14. Cengiz IF, Pitikakis M, Cesario L, Parascandolo P, Vosilla L, Viano G, Oliveira, JM, Reis, RL. Building the Basis for Patient-Specific Meniscal Scaffolds: From Human Knee MRI to Fabrication of 3D Printed Scaffolds. Bioprinting. 2016.
15. Pereira H, Cengiz IF, Silva-Correia J, Oliveira JM, Reis RL, Espregueira-Mendes J. Human meniscus: from biology to tissue engineering strategies. In: Doral MN, Karlsson J, editos. Sports Injuries. Springer; 2015. p. 1089-102.
16. Yan L-P, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012;8(1):289-301.
17. Yan L-P, Silva-Correia J, Oliveira MB, Vilela C, Pereira H, Sousa RA, Mano JF, Oliveira AL, Oliveira JM, Reis, RL. Bilayered silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: in vitro and in vivo assessment of biological performance. Acta Biomater. 2015;12:227-41.
18. Bacelar AH, Cengiz IF, Silva-Correia J, Sousa RA, Oliveira JM, L. RR. “Smart” Hydrogels in Tissue Engineering and Regenerative Medicine Applications. In: Khang G, editor. Handbook of Intelligent Scaffolds for Regenerative Medicine. Pan Stanford Publishing; 2015.
19. Buma P, Ramrattan N, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25(9):1523-32.
20. Reguzzoni M, Manelli A, Ronga M, Raspanti M, Grassi FA. Histology and ultrastructure of a tissue‐engineered collagen meniscus before and after implantation. J Biomed Mater Res B Appl Biomater. 2005;74(2):808-16.
21. Pereira H, Silva-Correia J, Oliveira J, Reis R, Espregueira-Mendes J. Future trends in the treatment of meniscus lesions: from repair to regeneration. In: Verdonk R, Espregueira-Mendes J, Monllau, JC, editors. Meniscal Transplantation. Springer; 2013. p. 103-12.
22. Stone KR, Steadman JR, Rodkey WG, Li S-T. Regeneration of Meniscal Cartilage with Use of a Collagen Scaffold. Analysis of Preliminary Data. J Bone Joint Surg Am. 1997;79(12):1770-7.
23. Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs E-L. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39(4):774-82.
24. Van Minnen B, Van Leeuwen M, Kors G, Zuidema J, Van Kooten T, Bos R. In vivo resorption of a biodegradable polyurethane foam, based on 1, 4‐butanediisocyanate: A three‐year subcutaneous implantation study. J Biomed Mater Res A. 2008;85(4):972-82.
25. Zuidema J, van Minnen B, Span M, Hissink C, van Kooten T, Bos R. In vitro degradation of a biodegradable polyurethane foam, based on 1, 4‐butanediisocyanate: A three‐year study at physiological and elevated temperature. J Biomed Mater Res A. 2009;90(3):920-30.
26. Pujol N, Verdonk P. Actifit Polyurethane Meniscus Scaffold: Basic Science, Techniques, and Results. In: Hulet C, Pereira H, Peretti G, Denti, M, editors. Surgery of the Meniscus. Springer; 2016. p. 543-51.
27. Hinarejos P, Erggelet C, Monllau JC. Collagen Meniscus Implant: Basic Science, Technique and Results. In: Hulet C, Pereira H, Peretti G, Denti, M, editors. Surgery of the Meniscus. Springer; 2016. p. 531-42.
28. Rodkey WG, DeHaven KE, Montgomery WH, Baker CL, Beck CL, Hormel SE, Steadman R, Cole B, Briggs K. Comparison of the collagen meniscus implant with partial meniscectomy. J Bone Joint Surg Am. 2008;90(7):1413-26.
29. Monllau JC, Gelber PE, Abat F, Pelfort X, Abad R, Hinarejos P, et al. Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy. 2011;27(7):933-43.
30. Hirschmann M, Keller L, Hirschmann A, Schenk L, Berbig R, Lüthi U, et al. One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):740-7.
31. Monllau JC. Collagen Meniscal Implant (CMI). In: Verdonk R, Espregueira-Mendes J, Monllau, JC, editors. Meniscal Transplantation. Springer; 2013. p. 73-82.
32. Implant MCM. Rehabilitation program summary for patients, surgeons, and physiotherapists. Franklin Lakes, NJ, USA: ReGen Biologics, Inc.; 2006.
33. Grassi A, Zaffagnini S, Muccioli GMM, Benzi A, Marcacci M. Clinical outcomes and complications of a collagen meniscus implant: a systematic review. Int Orthop. 2014;38(9):1945-53.
34. Zaffagnini S, Grassi A, Muccioli GMM, Bonanzinga T, Nitri M, Raggi F, Ravazzolo J, Marcacci, M. MRI evaluation of a collagen meniscus implant: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3228-37.
35. Filardo G, Andriolo L, Kon E, de Caro F, Marcacci M. Meniscal scaffolds: results and indications. A systematic literature review. Int Orthop. 2015;39(1):35-46.
36. Bulgheroni P, Bulgheroni E, Regazzola G, Mazzola C. Polyurethane scaffold for the treatment of partial meniscal tears. Clinical results with a minimum two-year follow-up. Joints. 2013;1(4):161.
37. Zaffagnini S, Muccioli GMM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, et al. Prospective Long-Term Outcomes of the Medial Collagen Meniscus Implant Versus Partial Medial Meniscectomy A Minimum 10-Year Follow-Up Study. Am J Sports Med. 2011;39(5):977-85.
38. Bulgheroni E, Grassi A, Bulgheroni P, Muccioli GMM, Zaffagnini S, Marcacci M. Long-term outcomes of medial CMI implant versus partial medial meniscectomy in patients with concomitant ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3221-7.
39. Zaffagnini S, Grassi A, Muccioli GMM, Holsten D, Bulgheroni P, Monllau JC, Berbig R, Lagae K, Crespo R, Marccaci M. Two-year clinical results of lateral collagen meniscus implant: a multicenter study. Arthroscopy. 2015;31(7):1269-78.
40. Gelber PE, Isart A, Erquicia JI, Pelfort X, Tey-Pons M, Monllau JC. Partial meniscus substitution with a polyurethane scaffold does not improve outcome after an open-wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):334-9..
| How to Cite this article: Bastos R, Andrade R, Pereira H, Oliveira JM, Reis RL, Rodeo S, Espregueira-Mendes J. Meniscal Scaffolds in the Clinics: Present and future trends.Asian Journal of Arthroscopy Aug – Nov 2016;1(2):47-52 . |





Leave a Reply
Want to join the discussion?Feel free to contribute!