Bertrand Sonnery Cottet, Sanesh Tuteja, Nuno Camelo Barbosa, Mathieu Thaunat
Volume 1 | Issue 2 | Aug – Nov 2016 | Page 28-34.
Author: Bertrand Sonnery Cottet[1], Sanesh Tuteja[1], Nuno Camelo Barbosa[1], Mathieu Thaunat[1].
[1] Investigation performed at the Centre Orthopédique Santy, FIFA medical center of Excellence, Groupe Ramsay-Générale de Santé, Lyon, France
Address of Correspondence
Dr Bertrand Sonnery-Cottet
Centre Orthopédique Santy, 24 Avenue Paul Santy, Lyon, 69008, France
Email: sonnerycottet@aol.com.
Abstract
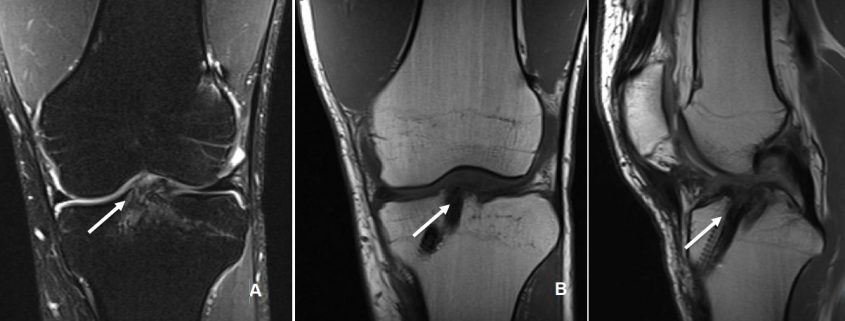
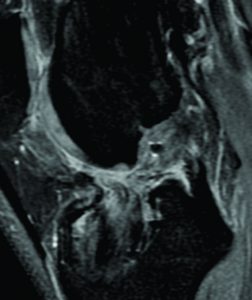
Ramp Lesions of the Medial Meniscus (MM) are associated with 9 to 17% of ACL Tears and are seldom recognized on preoperative magnetic resonance imaging (MRI) scans. They also often remain undiagnosed when viewing from the standard anterior compartment arthroscopic portals. Improved visualization is the key to achieving good meniscal repair results as it improves diagnosis of longitudinal tears in posterior horn MM, safeguards better debridement prior to repair and ensures good approximation of the torn ends under vision. A systematic posteromedial exploration allows discovery of and debridement of the hidden MM lesion and repair with a suture hook device is associated with low morbidity and must be undertaken whenever possible.
Keywords: Medial meniscus, Ramp lesion, Repair, Healing, Anterior cruciate ligament, Knee.
Introduction
Meniscal lesions of the posterior horn of medial meniscus (MM) are very often associated with an ACL rupture (16,32,49). Certain specific lesions of the Medial Meniscus (MM) such as meniscosynovial or meniscocapsular tears and meniscotibial ligament lesions are associated with 9 to 17% of ACL Tears (8,21) and are seldom recognized on preoperative magnetic resonance imaging (MRI) scans (8,38). They also often remain undiagnosed when viewing from the standard anterior compartment arthroscopic portals including a probing. They were named in the 1980s by Strobel et al (42) as ‘‘Ramp’’ lesions of the meniscus and have drawn a lot of attention over the past few years (3,8,21,38,42). The aim of this article will be to write a narrative review of this Ramp meniscal lesion.
History
Hamberg et al (15) first described “a peripheral vertical rupture in the posterior horn of the medial or lateral meniscus with an intact body” in 1983. They repaired these lesions through a postero-medial vertical arthrotomy; with the belief that the capillary blood supply from the capsule aids healing of the meniscus. They reported promising results (84% healing) in old and new lesions alike, thus providing some valuable insight into the philosophy of meniscal conservation. Morgan et al (25) in 1991 described the first arthroscopic vertical suture of the PHMM using Polydioxanone (PDS) sutures with an outside-to-inside technique. They reported a 16% failure rate occurring in patients with a concurrent ACL injury. They proposed that the rotation axis of the knee joint was altered in an ACL deficit knee thus placing excessive loads on the posterior horn of the medial meniscus. The kinematics of the posterior horn of the medial meniscus in the ACL deficient knee was therefore not conducive to meniscal healing after repair despite a peripheral blood supply. They also noted that, when combined with an ACL reconstruction, peripheral meniscal repair healing rates improved and approached those obtained in an ACL intact knee (25). Ahn et al (5) in 2004 described the first clinical series of an arthroscopic all-inside suture technique for tears in posterior horn of medial meniscus. Using a suture hook thorough 2-posteromedial portals, PHMM tears were repaired with concurrent reconstruction of the ACL. They concluded that the arthroscopic all-inside vertical suture using a hook resulted in a high healing rate even in large and complex vertical tears. Seil et al (38) in 2009 highlighted the indications for meniscal repairs based on the presence of associated ligamentous injuries and morphology of the lesion.
They advocated that Meniscal repairs be ‘‘ideally’’ carried out in:
1. Young patients (< 40 years)
2. No associated joint degeneration.
3. Vertical lesions in the peripheral third of the meniscus (3mm of the meniscosynovial junction) (4) and in conjunction with an ACL reconstruction.
4. Significantly displaced bucket-handle tear or an MMPH tear with vertical step off (5).
Epidemiology
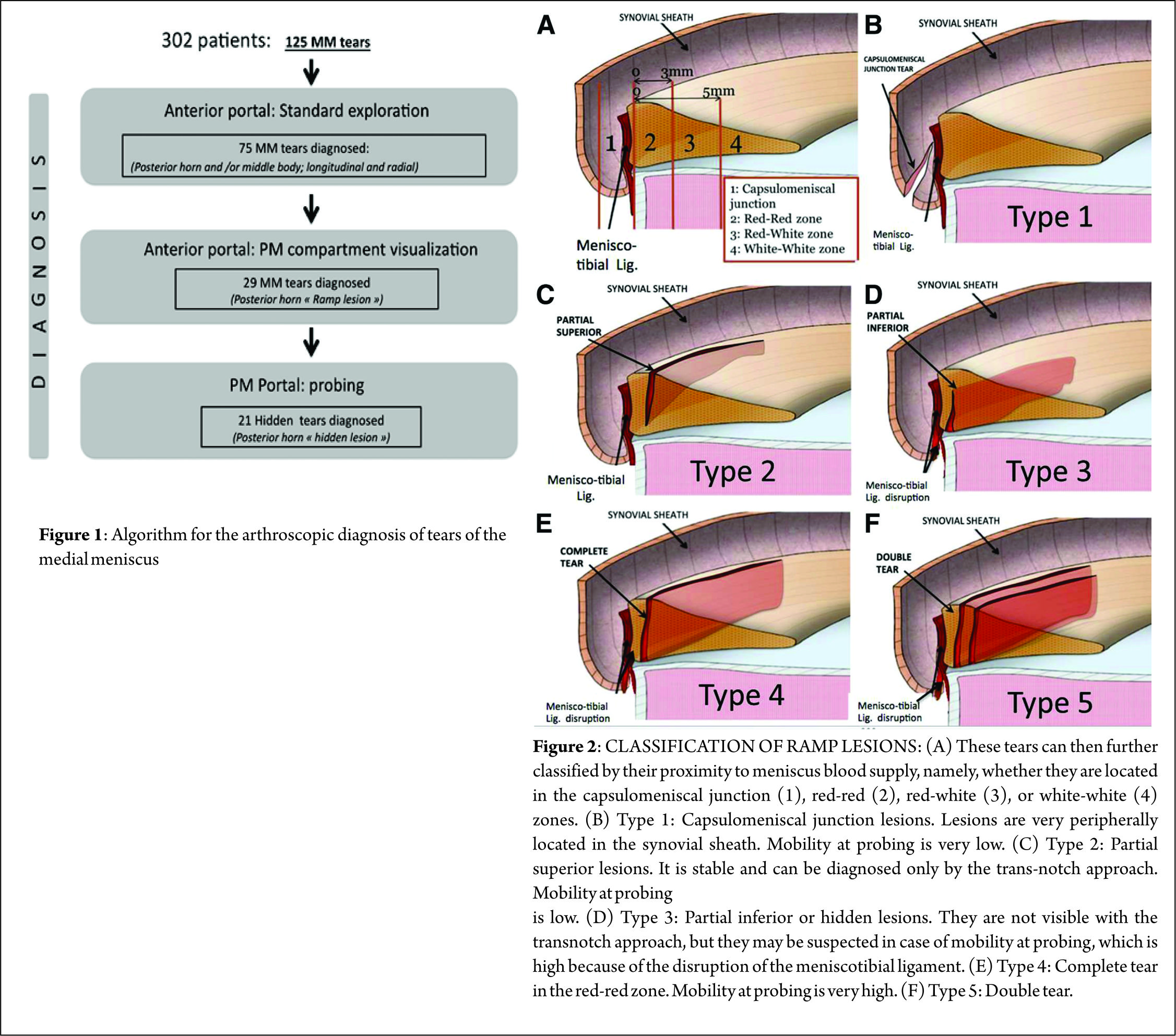
The prevalence for a meniscal lesion with an ACl tear has been reported between 47% to 61% (13,14). In 2010, S. Bollen et al (8) reported menisco-capsular lesions in 9.3% of their prospective series of 183 ACL reconstructions whereas Liu et al (21) described a prevalence of 16.6% in a series of 868 consecutive ACL reconstructions. In our series (40) on ACL deficient knees, a meniscal tears was identified in 125 out of the 302 patients. Following a systematic algorithm for exploration of the knee joint (Figure. 1), we found that; 75 (60%) medial meniscal body lesions were diagnosed through a standard anterior portal exploration, 29 (23.2%) ramp lesions were diagnosed during exploration of the posteromedial compartment, and 21 (16.8%) were discovered by probing the tear through a posteromedial portal and after minimal debridement of a superficial soft tissue layer with a motorized shaver. All-in-all, 42% (21/50) of the lesions diagnosed via inspecting the posterior compartment appeared only after superficial soft tissue debridement and were classified as ‘‘hidden lesions.’’ An ACL injury with an age at presentation above 30 years, male sex and a delay between injury and surgery are considered risk factors for concomitant meniscal lesions (8).
Biomechanical Implication on ACL
The importance of the meniscus in stabilizing the knee joint in chronically ACL-deficient knees has been validated by multiple studies (9, 39). A Peripheral posterior horn tear is caused by the recurrent trauma sustained by the Medial Meniscus, which acts as a ‘‘bumper’’ in ACL-deficient knees (2). In addition, contraction of the semimembranosus at its insertion along the posteromedial capsule may stress the peripheral meniscus, resulting in meniscocapsular tearing (17). This could occur at the time of injury or during subsequent instability episodes in the subacute or chronic situation (43). A capsular injury might also occur during the so-called medial contrecoup injury (18) after subluxation of the lateral tibial plateau and during subsequent reduction of the tibia (41). A longitudinal tear of the PHMM in ACL-deficient knees increases the anterior translation of the tibia and a repair of this lesion reduces Anteroposterior tibial translation significantly at most flexion angles (17) and most prominently at 30 degrees of flexion (12, 30). Peltier et al (30) observed that the PHMM was stabilised by the meniscotibial ligament posteriorly, which in turn inserted onto the posterior aspect of the proximal tibia. The capsule of the knee joint inserts more distally on this posterior surface. The posterior capsule hence lacks insertion onto the posterior aspect of the PHMM. Detachment of the ligament therefore results in an abnormal mobility of the entire PHMM, producing rotational instability. The authors observed that the division of the menisco-tibial ligament resulted in a statistically significant increase in internal tibial rotation. Such lesions occur either in the mid-substance (repairable) or as a bony avulsion (irreparable). Stephen et al (41) reported that the anterior tibial translation and external rotation were both significantly increased compared with the ACL-deficient knee after posterior meniscocapsular sectioning and these parameters were not restored after ACL reconstruction alone but were restored with ACL reconstruction combined with posterior meniscocapsular repair.
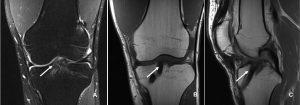
Classification [46] (Figure. 2)
We have proposed a classification for ramp lesions which is as follows:
Type 1: Ramp lesions. Very peripherally located in the synovial sheath. Mobility at probing is very low. (B)
Type 2: Partial superior lesions. It is stable and can be diagnosed only by trans-notch approach. Mobility at probing is low. ©
Type 3: Partial inferior or hidden lesions. It is not visible with the trans-notch approach, but it may be suspected in case of mobility at probing, which is high. (D)
Type 4: Complete tear in the red-red zone. Mobility at probing is very high. (E)
Type 5: Double tear.
Surgical Technique (46)
With the patient supine on the operating table, a tourniquet is placed high on the thigh, and the knee placed at 90º of flexion with a foot support to allow full range of knee motion. Using standard arthroscopy portals, high lateral as viewing and medial portal for instrumentation, articular inspection is performed and we engage a probe in the posterior segment of the meniscus and force an anterior excursion of the meniscus. If the meniscus subluxates under the condyle, it is an indicator for instability and an indirect sign of a ramp lesion. Direct visualization of the posteromedial compartment is mandated to diagnose and repair these lesions. Even if the meniscus appears stable on probing, a systematic exploration of the posterior segment must be performed using the protocol in (Figure.1) Through a Guillquist maneuver, the arthroscope in the anterolateral portal is advanced in the triangle formed by the medial femoral condyle, the posterior cruciate ligament, and the tibial spines. With valgus force applied initially in flexion followed by knee extension, the arthroscope is pass through the space at the condyle border of the medial femoral condyle. Internal rotation applied to the tibia further enhances visualization; this causes subluxation of the posterior tibial plateau causing a posterior translation of the middle third segment. Almost two-thirds of peripheral lesions can be diagnosed with this maneuver. Tears of the posterior segment must be approached posteromedially. The posteromedial portal is placed superior to the hamstring tendons and posterior to the medial joint line, also trans illumination allows observation of the great saphenous vein, that is in close relation to the infra-patellar branch of the internal saphenous nerve, that must be avoided. The needle is introduced from outside to inside, in the direction of the lesion. The portal is prepared with a number 11blade scalpel under arthroscopic control. The all-inside suture is accomplished with or without a working cannula, depending on the surgeons choice. Using a shaver the lesion is debrided and the intervening fibrous tissue is excised. Suturing is carried out using a 25º curved hook (Suture Lasso, Arthrex): a left curved hook is used for a right knee and vice versa. The curved hook is loaded with a no. 2 non-absorbable braided composite suture (Fiberwire, Arthrex) or a absorbable no.1 PDS introduced through the posteromedial portal. The curved hook must penetrate the peripheral wall of the MM, and then the inner wall of the MM. The free end of the suture in the posteromedial space is grasped and brought out through the posteromedial portal. A sliding knot (fishing knot type) is applied to the most posterior part of the meniscus with the help of a knot pusher and then cut. The sutures are repeated as required depending on the length of the tear (usually we place a knot every 5 mm of the tear). During suturing, care must be taken to not splinter the meniscus that can occur with multiple failed attempts to pass the curved hook. Additionally, entangling of the sutures must be avoided. In some patients, the tear may extend to the mid-portion of the meniscus requiring further repair through the standard anterior portal with meniscal suture anchor and/or an outside-in suture. The stability of the suture is then tested with the probe.
Post-operative
Post operatively, active and passive range of motion is limited to 0-90º in the first six weeks. Full weight bearing is allowed by six weeks post-operatively. Jogging is permitted after 4 months, pivoting activity at 6 months, and unrestricted activities by 9 months.
Results
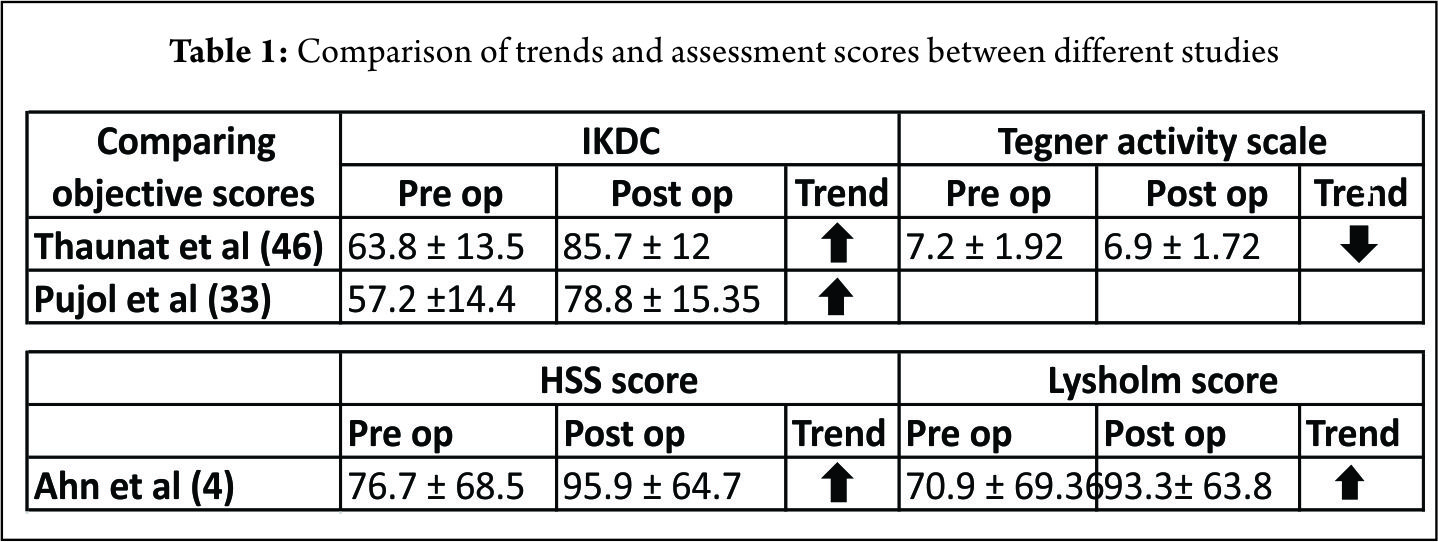
Ahn et al (4) in 140 patients showed complete healing in 118, incomplete healing in 17 and failure 5 repairs. The clinical success rate was 96.4% (135 of 140). Healing was associated with the type of tear and location. Incomplete healing and failures had complex tears or tears involving the red-white zone. Seventeen patients had incomplete healing at second-look arthroscopy but had no clinical sign of a meniscal tear. The mean Lysholm score and HSS scores improved post surgery (Table. 1) and 134 (95.7 %) patients had a normal (104 patients, 74.3%) or nearly normal (30 patients, 21.4%) the objective IKDC scores. In our prospective evaluation (46) of 132 patients undergoing a MM repair through a posteromedial portal in conjunction with ACL reconstruction, fifteen patients were found to be symptomatic according to Barret’s criteria on follow-up (6). The clinical failure rate was 9%, 4.9% in the subgroup “limited tear” and 15.7% in the subgroup “extended tear.” A limited tear was defined as one restricted to the posterior segment whereas those with a tear that extended to the mid-portion of the meniscus were classified as an extended tear. The extended tears required an additional repair through the standard anterior portal with meniscal suture anchor and/or an outside-in suture. The extended lesions had an increased risk of clinical failure. Out of the fifteen, 9 patients underwent revision surgery. Nine patients (6.8%) had failure of the meniscal repair; 3.7% (3/81) occurred in the subgroup of limited tears and 11.7% (6/51) in the subgroup extended tears. In the subgroup of extended tears, the cumulative survival rate did not decrease significantly and were not associated with a significant increased risk of revision of the MM. The average subjective IKDC improved at last follow-up and The Tegner activity scale at the last follow-up was slightly lower than before surgery (Table. 1).
Complications
The main complications may be related to the posteromedial portal placement. Damage to the infra-patellar branch of the saphenous nerve due to a posteromedial portal has been reported owing to the proximity of the nerve to the portal site causing hypoesthesia or paresthesia below the patella (27). Having said that, hypoesthesia resulting from harvesting the Semi-tendinous and Gracilis tendons in a concomitant ACL reconstruction may be responsible for 74% of the times (35). Transient hypoesthesia of the Sartorial branch of the saphenous nerve has also been reported probably due to an access portal situated too anteriorly (24). McGinnis et al (23) studied the neurovascular safety zone for the posteromedial access and recommended a portal through the posterior soft spot located formed by the medial head of the gastrocnemius, the tendon of the semimembranosus and the medial collateral ligament at the posterior aspect of the joint line for creation of the posteromedial portal. Hemarthrosis due to the long saphenous vein injury may occur in the postero medial approach (27). Among other complications, an iatrogenic medial meniscus tear may occur from repeated attempts at suturing the meniscus with a curved hook, rending suture impossible. Also, to our knowledge no popliteal artery, common peroneal and tibial nerve lesions has been reported, however they are at risk of damage during creation of the posterior portals. These complications may be avoided by placing the posterior portals with knee in 90 degrees of flexion. This moves the neurovascular structures posteriorly, away from the posteromedial portal site. Also, the Guillquist maneuver that provides trans-illumination may help visualize the course of the superficial veins and the accompanying nerves thus preventing inadvertent damage (35). Our series (46) also has a low complications rate with only two cases of hemarthrosis post operatively. Also, no patient developed a neuroma around the location of the posteromedial approach, although it was difficult to be accurately determining the incidence of saphenous nerve lesions due to the posteromedial approach as the hamstring tendon harvesting can cause hypoesthesia in the different territories of the saphenous nerve.
Discussion
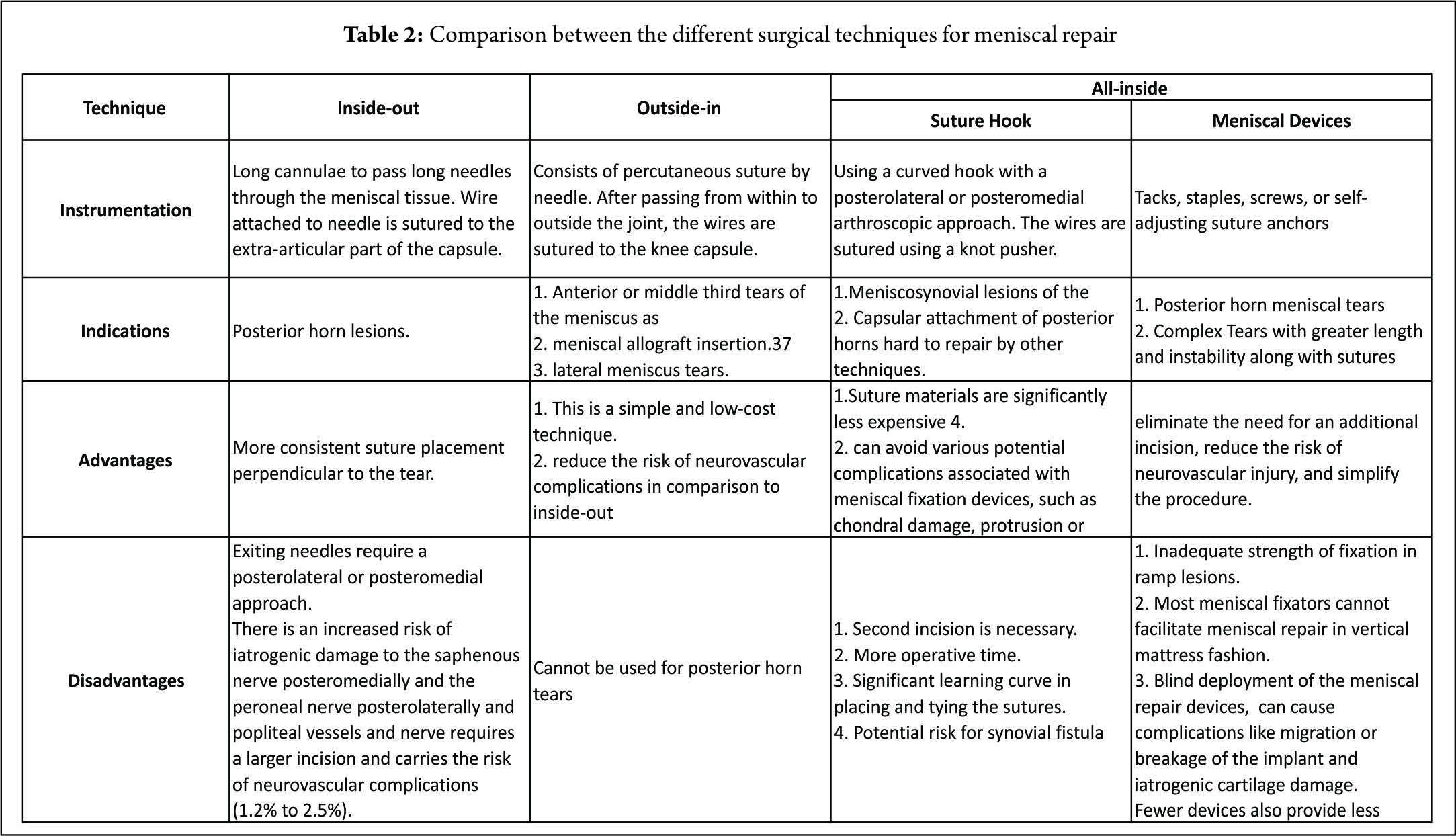
The forces acting on the MM increase by as much as 200% after an ACL injury. Furthermore, forces acting on the ACL replacement graft increase by 33% to 50% after a medial meniscectomy (12,28). Deficiency of the medial meniscus has therefore been proposed as a secondary cause of ACL failure. It has thus been recommended that an ACL-deficient knee be reconstructed to protect the menisci (39,50). Conversely, identification and repair of a ramp lesion during an ACL reconstruction is imperative to reduce the risk of secondary graft failures, as these lesions may increase the anterior tibial translation (2,12, 30) and subsequently the strain on the graft. The success rates for meniscal repairs have been reported to be from 70% to 90% in vascular regions (11,13,16,36). Anh et al (12) reported a clinical successful healing rate of 96.4% in PHMM repairs with concomitant ACL reconstruction. Tenuta and Arciero (45) reported higher healing rates in concomitant ACL reconstruction than for isolated repairs (90% vs 57%). Meniscal repair in conjunction with ACL reconstruction has been reported to create a favorable environment for meniscal healing because of fibrin clot formation associated with intra-articular bleeding generated during ACL reconstruction (44). Multiple techniques for suturing the meniscus are available. The indications, advantages and disadvantages of each are mentioned in (Table.2). The all-inside suture repair technique using a hook is especially useful in a ramp lesion, as the use of newer devices makes the repair procedure blind and placing a suture in the vertical configuration is technically challenging. In addition Choi et al reported that the use of meniscal devices failed to provide sufficient strength of fixation. They recommended that during suturing, the posteromedial capsule should be elevated and approximated to the PHMM to ensure precise approximation of tear site (10). In spite of the development of the newer all-inside suture devices, the failure rate of the repair of PHMM tears continues to remains high, (19) which may be attributed to various factors that include inadequate visualization and debridement of the lesions of the PHMM; failure to confirm the reduction of the lesion with the all inside technique (48) and tissue failure due suture pullout through the meniscal tissue (45).
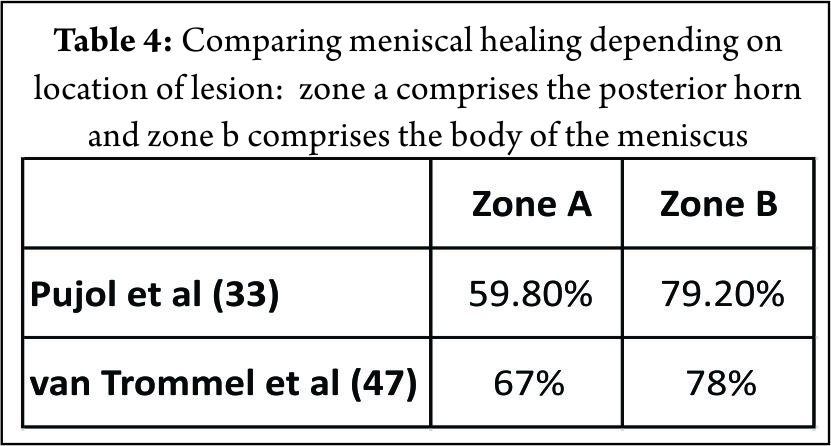
The mechanical strength of the vertical suture is greater than that of the horizontal suture (45). Having said that, most meniscal fixators cannot facilitate meniscal repair in a vertical mattress fashion (7,34) especially in the posteromedial corner of the medial meniscus, small or tight knee joints. Sutures spaced at every 3 to 5 mm have been recommended however; the optimal number is unknown (44). Pujol et al (33) using meniscal devices reported an overall healing rate of 73.1%. van Trommel et al (47) reported similar results (76%) with the outside-in technique. (Table. 3) Both studies observed a strong trend toward a relatively lower healing rate of the posterior horn (Zone A), as compared with the body (Zone B). (Table. 4) They also observed that, partial healing in all tears extending from the posterior to the middle third of the medial meniscus. We observed similar results in our study with a higher failure rate in the extended tear subgroup (6/51) (46). Pujol et al (33) attributed this to the difficulty in performing an adequate abrasion of the posterior segment using standard anterior arthroscopic portals whereas van Trommel et al (47) attributed that same to the relatively anterior placement of the needles with the outside-in technique, making a perpendicular repair extremely difficult. This resulted in a decrease in the coaptation force of the sutures. In addition, they observed that an oblique suture placement in the posterior zone with the outside-in technique made the sutures enter more anterior than they exit. Ahn et al (5) postulated that a torn posterior menisco-capsular structure moved inferiorly against the remaining meniscus, displacing the tear during knee flexion. They suggested that this motion of the torn medial meniscus can partially explain the slow healing observed in MMPH peripheral rim tears despite a rich vascular supply to the red-red zone. Pujol et al (31) in 2011 reported a secondary meniscectomy rate of 12.5%. The authors observed that the volume of meniscus removed decreased in 35% of cases, with respect to the initial tear and noted that a secondary meniscectomy following repair can partially save the meniscus and the failure called a ‘‘partial’’ failure. They recommended that suturing a tear therefore preserved the meniscal volume in a subsequent meniscectomy performed for a failure of repair or repeat tears. Tachibana et al (44) reported newly formed meniscal tears occurring in an area different from the initial repair site, on the surface of 34.5% of the healed and incompletely healed menisci. These new injuries were 1 to 3 mm in length partial- or full-thickness lesions and located central to the peripheral repair. In our series (46), the high rate of recurrent tear was as a result of newly formed tears that were confirmed on the surface of 5 menisci. It is conceivable that these injuries were attributable to a residual cleft left by the path of the Suture Lasso and maintained by the use of a strong no. 2 non-absorbable suture.

These clefts on the avascular meniscal substance may remain in situ without healing and would favor the recurrence of a more centrally located lesion in the white-white zone. Using a small suture hook device may therefore be desirable as it may reduce the size of the clefts created during suturing. In addition, the ‘cheese wiring effect’ due to the higher co-efficient of friction of a non-absorbable suture may contribute to a failure. We therefore decided to change our suture from a strong non-absorbable suture to a number 0 or 1 PDS suture, which are recommended to reduce the risk of these newly formed injury (37).
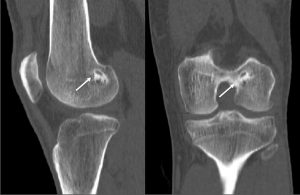
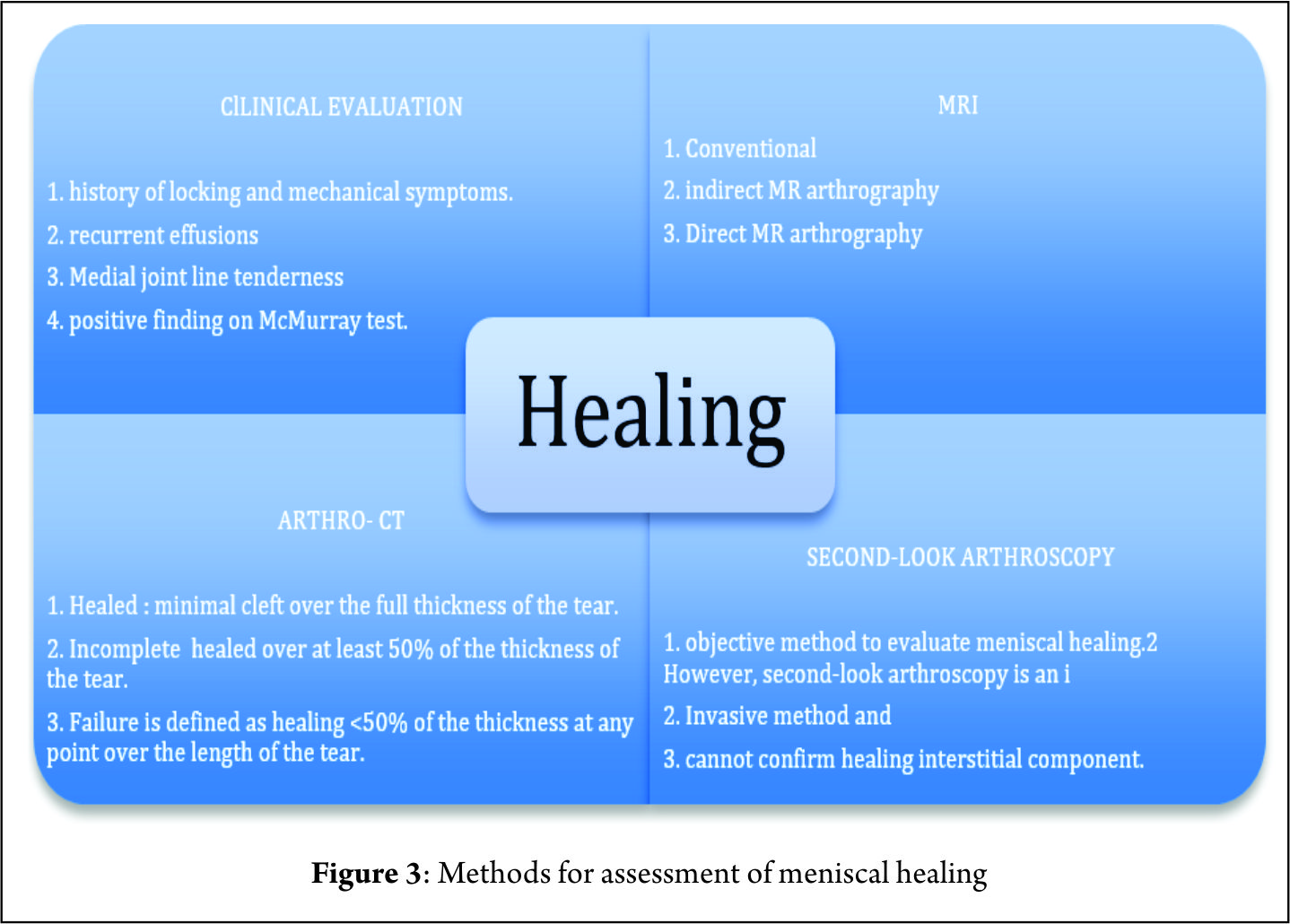
Nepple et al (26) observed that the time between injury and repair was the most important factor influencing healing. The zone of tear in reference to blood supply is another major factor affecting the results of a meniscal repair and ramp lesions in the red-red zone are expected to heal more readily than are those in the red-white zone (20). The criteria for healing based on follow-up arthro-CT corresponding to thickness of healing was suggested by Henning et al (36) can supplement clinical evaluation to improve diagnostic accuracy (Figure 3). The clinical failure rate in a systematic review ranged from 0% to 43.5%, with a mean failure rate of 15% (22). Failures after two years represented nearly 30% (26). Although numerous studies have reported short-term outcomes of various techniques of meniscal repair, relatively few have reported medium to long-term outcomes. The rate of meniscal repair failure appears to increase from short-term follow-up to medium to long-term follow-up regardless of the technique (26). There are limited numbers of studies assessing the outcomes of meniscal repair using the PM approach (4,5,10,46). Further prospective analysis with long – term follow-up is required to validate the promising early results of meniscal repairs performed with this approach. Finally, improved visualization is the key to achieving good meniscal repair results as it improves diagnosis of longitudinal tears in posterior horn MM (30), safeguards better debridement prior to repair and ensures good approximation of the torn ends under vision (1). It is thus important to perform a systematic exploration of the knee during an ACL reconstruction (Figure 1). A transnotch visualization combined with palpation of the meniscus with a needle or probe through the postero-medial portal aids in diagnosis of ramp lesions, which may otherwise be missed. Hidden lesions furthermore may be either very peripheral, covered by a layer of synovial or scar tissue or may not be reachable with a probe. It is therefore essential to identify these lesions during an ACL reconstruction and repair them whenever they are found to be unstable (40).
Conclusions
A systematic posteromedial exploration allows discovery of and debridement of the hidden MM lesion and repair with a suture hook device is associated with low morbidity. Failure Rates following a ramp lesion repair are low and occurs during the first 20 months. Even if a failure occurs the subsequent meniscectomy is limited and the volume of meniscal tissue debrided is reduced. An arthroscopic repair of meniscal ramp lesions should therefore be undertaken whenever possible.
References
1. Ahldén M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J. The Swedish National Anterior Cruciate Ligament Register A Report on Baseline Variables and Outcomes of Surgery for Almost 18,000 Patients. The American journal of sports medicine. 2012 Oct 1;40(10):2230-5.
2. Ahn JH, Bae TS, Kang KS, Kang SY, Lee SH. Longitudinal Tear of the Medial Meniscus Posterior Horn in the Anterior Cruciate Ligament–Deficient Knee Significantly Influences Anterior Stability. The American journal of sports medicine. 2011 Oct 1;39(10):2187-93.
3. Ahn JH, Kim SH, Yoo JC, Wang JH. All-inside suture technique using two posteromedial portals in a medial meniscus posterior horn tear. Arthroscopy. 2004;20:101-108.
4. Ahn JH, Lee YS, Yoo JC, Chang MJ, Koh KH, Kim MH. Clinical and second-Look arthroscopic evaluation of repaired medial meniscus in anterior cruciate ligament–reconstructed knees. The American journal of sports medicine. 2010 Mar 1;38(3):472-7.
5. Ahn JH, Wang JH, Yoo JC. Arthroscopic all-inside suture repair of medial meniscus lesion in anterior cruciate ligament-deficient knees: results of second-look arthroscopies in 39 cases. Arthroscopy. 2004;20(9):936-45.
6. Barrett GR, Field MH, Treacy SH, Ruff CG. Clinical results of meniscus repair in patients 40 years and older. Arthroscopy 1998;14:824-829.
7. Boenisch UW, Faber KJ, Ciarelli M, Steadman JR, Arnoczky SP. Pull-out strength and stiffness of meniscal repair using absorbable arrows or Ti-Cron vertical and horizontal loop sutures. The American journal of sports medicine. 1999 Sep 1;27(5):626-31.
8. Bollen SR. Posteromedial meniscocapsular injury associated with rupture of the anterior cruciate ligament: a previously unrecognized association. J Bone Joint Surg Br. 2010;92:222-223.
9. Bonnin M, Carret JP, Dimnet J, Dejour H. The weight-bearing knee after anterior cruciate ligament rupture: an in vitro biomechanical study. Knee Surg Sports Traumatol Arthrosc. 1996;3:245-251.
10. Choi NH, Kim TH, Victoroff BN. Comparison of Arthroscopic Medial Meniscal Suture Repair Techniques Inside-Out Versus All-Inside Repair. The American journal of sports medicine. 2009 Nov 1;37(11):2144-50.
11. DeHaven KE. Meniscus repair. The American journal of sports medicine. 1999;27:242-250.
12. Edgar C Hartford W, Ware, Obopilwe, E Ziegler C, Reed D, Arciero R. Posteromedial Meniscocapsular Tear: Prevalence, Detection Sensitivity, Biomechanics, and Repair Technique. Scientific Exhibit SE81. AAOS 2015.
13. Feng H, Hong L, Geng XS, Zhang H, Wang XS, Jiang XY. Secondlook arthroscopic evaluation of bucket-handle meniscus tear repairs with anterior cruciate ligament reconstruction: 67 consecutive cases. Arthroscopy. 2008;24:1358-1366.
14. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Intraoperative findings and procedures in culturally and geographically different patient and surgeon populations: an anterior cruciate ligament reconstruction registry comparison between Norway and the USA. Acta Orthop. 2012;83:577-582.
15. Hamberg P, Gillquist J, Lysholm JA. Suture of new and old peripheral meniscus tears. J Bone Joint Surg Am. 1983 Feb 1;65(2):193-7.[9]
16. Henning CE. Current status of meniscus salvage. Clin Sports Med. 1990;9:567-576.
17. Ihara H, Miwa M, Takayanagi K, Nakayama A. Acute torn meniscus combined with acute cruciate ligament injury: second look arthroscopy after 3-month conservative treatment. Clin Orthop Relat Res. 1994;307:146-154.
18. Kaplan PA, Walker CW, Kilcoyne RF, Brown DE, Tusek D, Dussault RG. Occult fracture patterns of the knee associated with anterior cruciate ligament tears: assessment with MR imaging. Radiology. 1992;183(3):835-838.
19. Kotsovolos ES, Hantes ME, Mastrokalos DS, Lorbach O, Paessler HH. Results of all-inside meniscal repair with the FasT-Fix meniscal repair system. Arthroscopy. 2006;22(1):3-9.
20. Krych AJ, McIntoch AL, Voll AE, Stuart MJ, Dahm DL. Arthroscopic repair of isolated meniscal tears in patients 18 years and younger. Am J Sports Med. 2008;36:1283-1289.
21. Liu X, Feng H, Zhang H, Hong L, Wang XS, Zhang J. Arthroscopic prevalence of ramp lesion in 868 patients with anterior cruciate ligament injury. The American journal of sports medicine. 2011;39:832-837.
22. Lozano J, Ma CB, Cannon WD. All-inside meniscus repair: a systematic review. Clin Orthop Relat Res. 2007;455:134-141.
23. McGinnis MD, Gonzalez R, Nyland J, Caborn DN. The posteromedial knee arthroscopy portal: a cadaveric study defining a safety zone for portal placement. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2011 Aug 31;27(8):1090-5.
24. Morgan CD, Casscells SW. Arthroscopic meniscus repair: a safe approach to the posterior horns. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1986 Dec 31;2(1):3-12.
25. Morgan CD, Wojtys EM, Casscells CD, Casscells SW. Arthroscopic meniscal repair evaluated by second-look arthroscopy. The American journal of sports medicine. 1991 Dec 1;19(6):632-8.
26. Nepple JJ, Dunn WR, Wright RW. Meniscal repair outcomes at greater than five years: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012 Dec 19;94(24):2222-7.
27. Ogilvie-Harris DJ, Biggs DJ, Mackay M, Weisleder L. Posterior portals for arthroscopic surgery of the knee. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1994 Dec 31;10(6):608-13.
28. Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med. 2001;29:226-231.
29. Papastergiou SG, Koukoulias NE, Mikalef P, Ziogas E, Voulgaropoulos H. Meniscal tears in the ACL-deficient knee: correlation between meniscal tears and the timing of ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:1438-1444.
30. Peltier A, Lording T, Maubisson L, Ballis R, Neyret P, Lustig S. The role of the meniscotibial ligament in posteromedial rotational knee stability. Knee Surgery, Sports Traumatology, Arthroscopy. 2015 Oct 1;23(10):2967-73.
31. Pujol N, Barbier O, Boisrenoult P, Beaufils P. Amount of meniscal resection after failed meniscal repair. The American journal of sports medicine. 2011 Aug 1;39(8):1648-52.
32. Pujol N, Beaufils P. Healing results of meniscal tears left in situ during anterior cruciate ligament reconstruction: a review of clinical studies. Knee Surg Sports Traumatol Arthrosc. 2009;17:396-401.
33. Pujol N, Panarella L, Selmi TA, Neyret P, Fithian D, Beaufils P. Meniscal Healing After Meniscal Repair A CT Arthrography Assessment. The American journal of sports medicine. 2008 Aug 1;36(8):1489-95.
34. Rankin CC, Lintner DM, Noble PC, Paravic V, Greer E. A biomechanical analysis of meniscal repair techniques. Am J Sports Med. 2002;30:492-497.
35. Sanders B, Rolf R, McClelland W, Xerogeanes J. Prevalence of saphenous nerve injury after autogenous hamstring harvest: an anatomic and clinical study of sartorial branch injury. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2007 Sep 30;23(9):956-63.
36. Scott GA, Jolly BL, Henning CE. Combined posterior incision and arthroscopic intra-articular repair of the meniscus: an examination of factors affecting healing. J Bone Joint Surg Am. 1986;68: 847-861.
37. Seil R, Rupp S, Jurecka C, Rein R, Kohn D. Effect of various suture strength factors on behavior of meniscus sutures in cyclic loading conditions. Unfallchirurg 2001;104:392-398.
38. Seil R, VanGiffen N, Pape D. Thirty years of arthroscopic meniscal suture: what’s left to be done? Orthop Traumatol Surg Res. 2009;95:S85-S96.
39. Shoemaker SC, Markolf KL. The role of the meniscus in the anteriorposterior stability of the loaded anterior cruciate-deficient knee: effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71-79.
40. Sonnery-Cottet B, Conteduca J, Thaunat M, Gunepin FX, Seil R. Hidden Lesions of the Posterior Horn of the Medial Meniscus A Systematic Arthroscopic Exploration of the Concealed Portion of the Knee. The American journal of sports medicine. 2014 Feb 24:0363546514522394.
41. Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA. Posteromedial Meniscocapsular Lesions Increase Tibiofemoral Joint Laxity With Anterior Cruciate Ligament Deficiency, and Their Repair Reduces Laxity. Am J Sports Med. 2016 Feb;44(2):400-8. doi: 10.1177/0363546515617454. Epub 2015 Dec 11.
42. Strobel MJ. Menisci. In: Fett HM, Flechtner P, eds. Manual of Arthroscopic Surgery. New York: Springer; 1988:171-178.
43. Sullivan D, Levy IM, Sheskier S, Torzilli PA, Warren RF. Medial restraints to anterior-posterior motion of the knee. J Bone Joint Surg Am. 1984;66:930-936.
44. Tachibana Y, Sakaguchi K, Goto T, Oda H, Yamazaki K, Iida S. Repair integrity evaluated by second-look arthroscopy after arthroscopic meniscal repair with the FasT-Fix during anterior cruciate ligament reconstruction. The American journal of sports medicine. 2010 May 1;38(5):965-71.
45. Tenuta JJ, Arciero RA. Arthroscopic evaluation of meniscal repairs: factors that effect healing. Am J Sports Med. 1994;22:797-802.
46. Thaunat M, Jan N, Fayard JM, Kajetanek C, Murphy CG, Pupim B, Gardon R, Sonnery-Cottet B. Repair of Meniscal Ramp Lesions Through a Posteromedial Portal During Anterior Cruciate Ligament Reconstruction: Outcome Study With a Minimum 2-Year Follow-up. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016 May 13.
47. van Trommel MF, Simonian PT, Potter HG, Wickiewicz TL. Different regional healing rates with the outside-in technique for meniscal repair. The American journal of sports medicine. 1998 May 1;26(3):446-52.
48. Walgrave S, Claes S, Bellemans J. High incidence of intraoperative anchorage failure in FasT fix all inside meniscal suturing device. Acta Orthop Belg. 2013;79(6):689-93.
49. Warren RF, Marshall JL. Injuries of the anterior cruciate and medial collateral ligaments of the knee: a long-term follow-up of 86 cases– part II. Clin Orthop Relat Res. 1978;136:198-211.
50. Yoo JC, Ahn JH, Lee SH, Yoon YC. Increasing incidence of medial meniscal tears in nonoperatively treated anterior cruciate ligament insufficiency patients documented by serial magnetic resonance imaging studies. Am J Sports Med. 2009;37:1478-1483.
| How to Cite this article:. Sonnery-Cottet B, Tuteja S, Barbosa NC, Thaunat M. Meniscus Ramp Lesion. Asian Journal of Arthroscopy Aug – Nov 2016;1(2):28-34. |