Vijay D Shetty, Karan Alva, Varun Gupta
Volume 1 | Issue 1 | April – Jun 2016 | Page 25-28
Author: Vijay D Shetty [1], Karan Alva [1], Varun Gupta [1]
[1] LH Hiranandani Hospital, Hiranandani Orthopaedic Medical Education (HOME), Mumbai 400 076,India.
Address of Correspondence
Dr Vijay D Shetty
Dr LH Hiranandani Hospital
Hiranandani Orthopaedic Medical Education (HOME)
Mumbai 400 076 India
Email id – vijaydshetty@gmail.com
Abstract
The success of anterior cruciate ligament reconstruction depends on the healing and integration of the graft in the bony tunnels. Recently there has been lot of interest in biological augmentation techniques to improve the biological milieu in the knee joint so as to enhance this healing process. These techniques include use of platelet rich plasma, periosteum and bone morphogenetic proteins (BMPs). The present article is a review of current literature exploring the effectiveness of these techniques and the future scope
Keywords: anterior cruciate ligament reconstruction, platelet rich plasma, bone morphogenetic proteins
Introduction
The anterior cruciate ligament (ACL) is the most commonly injured knee ligament, frequently requiring surgery and extensive rehabilitation(1). Primary repair of the ACL has a high failure rate of 40% to 100% mandating the need for ACL reconstruction (ACLR)(2). However, success of ACLR depends mainly on the healing and integration of graft into the femoral and tibial tunnels(3,4,5). Various factors such as graft selection, graft incorporation and pre-injury activity level influence the clinical outcome of ACLR(6,7). Studies have shown that younger the age of the patient and, more athletic the demand, higher is the expectation from the surgery(8,9).
Graft-bone healing has been always an issue with ACLR. Recent years have seen a number of publications indicating the use of biological augmentation techniques to enhance graft-bone healing(10,11). These include the use of platelet rich plasma, periosteum and bone morphogenetic proteins (BMPs). This article attempts to explore the current thinking in the use of biologics in enhancing bone-graft healing in ACLR.
Graft healing
Natural healing of torn ACL is one of the challenging problems encountered by surgeons. The hypo-vascular and hypo-cellular nature of ACL retards its self regeneration capacity and results in poor healing. Further, following ACL injury, the thin synovial sheath surrounding the ACL gets disrupted resulting in mixing of blood with the native synovial fluid. As a result, haematoma formation is delayed and this prevents the aggregation of factors (cytokines, growth factors and reparative cells) responsible for natural healing(12). This forms the basis of non-healing of injured ACL. Therefore, the best option to address this issue would be a reconstruction rather than repair.
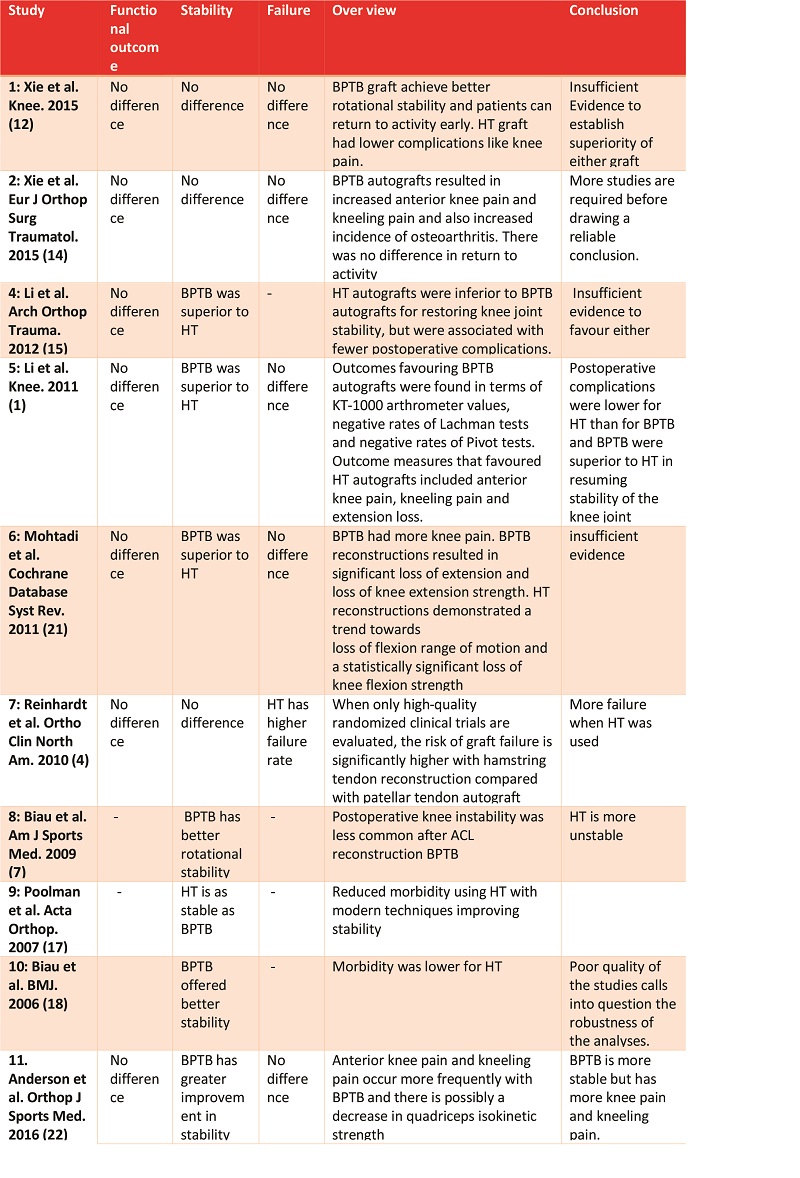
Normally, ACL inserts directly into the bone, thus forming a transition zone from the tendon-to-bone consisting of the tendon, non-mineralized fibrocartilage, mineralized fibrocartilage and bone (Figure 1). The fibrocartilage at the insertion site contains cartilage-specific collagens, type II, IX, X and XI, with the interface between mineralized and demineralized bone maintained by collagen X(13). Besides, obliquely running Sharpey fibres are present at insertion sites which anchors the ligament to bone, providing the fundamental mechanical strength. ACLR with tendon grafts fails to reproduce the same arrangement. It has been shown by various studies that graft healing in ACLR occurs by an interposed layer of fibro-vascular scar tissue at the graft tunnel site(14). This fibro-vascular scar tissue becomes mineralized and incorporates the tendon graft into the surrounding bone. The tendon bone junction is restored by the re-growth of collagen fibres between the tendon and bone(15,16,17,18,19). The formation of collagens and Sharpey fibres occurs after 6 weeks of surgery and bone tunnel healing of graft is completed by 6-10 months after surgery(20). However poor osteo-integration of the graft in ACLR is common and is associated with anterior –posterior laxity postoperatively (21). Thus, to achieve earlier return to functional activities and better clinical outcomes, acceleration of healing between tendon graft and bone is the most enduring challenge. In order to improve graft bone healing, various biologically engineered strategies are being studied. These biological strategies aim to enhance intra-articular and intra-osseous healing.
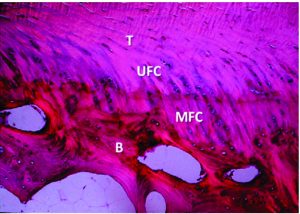
Figure 1: Organised transition from tendon (T) to demineralised fibrocartilage (UFC) to mineralized fibrocartilage (MFC) and bone (B). Courtesy: Muller et al, 2013.
Figure 2: The periosteum is wrapped with the cambium layer facing the tunnel wall and then sutured on the tendon at both sides with a No. 3-0 Vicryl suture where the tendon graft approached the tunnel opening. Courtesy: Chen at al, 2010.
Biological strategies to enhance tendon graft healing in ACLR
Platelet-rich plasma
Platelet-rich plasma (PRP) is an autologous concentration of platelets. The concentration of these platelets, in a given formulation, is much above the normal physiological levels. Platelets are the precursors of the megakaryocytes having an irregular shape with non-nucleated cytoplasmic bodies. The glycoprotein’s expressed on their cell membranes play an important role in haemostasis and wound healing by formatting fibrin clots(22). Platelets are the source of various growth factors including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-beta 1 (TGF-β1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and insulin-like growth factor (IGF-I) which are involved in different stages of cell proliferation(23,24,25).
Versatility of PRP lies in the fact that it can be easily prepared and can be applied directly in the operation theatre. Various methods of its application are intra- articular injection or in the form of a membrane that can be applied directly to target site. Several studies have shown that the healing potential of PRP in articular tissues, cartilage, ligaments, tendons and synovium(26,27,28). Infiltration of PRP causes increase in the extracellular matrix deposition, anabolic reaction towards cells, reduction of pro apoptotic signals and also has anti–inflammatory effect in the joint environment(26). Studies have shown that application of PRP in ACL reconstruction procedure not only causes better and faster ligamentization of the graft, but also contributes to a better integration of the graft within the bone tunnels. Enlargement of bone tunnels can thus be prevented and faster healing can be promoted.
Periosteum
Periosteum is a bilayered tissue between the bone and soft tissue. It had an outer fibrous layer which is rich in fibroblasts while the inner cambium layer is rich in multipotent mesodermal cells. It also consists of chondro-progenitor and osteo-progenitor cells, which can differentiate into both cartilage and bone(29,30). It can be easily harvested at the proximal tibia from a routine incision for hamstring tendon harvesting (Figure 2). The tendon reconstruction is said to be successful if there is bony in-growth into the tendon.8 The periosteum may be used to enhance the healing between the bone and graft by forming fibrocartilage and calcified fibrocartilage(31,32). Besides, it can also help to seal the intra-articular tunnel opening in the early postoperative period, thus avoiding synovial fluid reflux into the tunnel(33,34). Studies have shown favourable outcome with the use of periosteum to enhance tendon-bone healing post ACLR. In a study by Chen et al, (31) knees were followed up for a mean of 4.6 years post ACLR which showed statistically significant results with periosteum-enveloping hamstring tendon single bundle ACLR when compared to other studies with comparable fixation(35).
Bone morphogenetic protein
Bone morphogenetic proteins (BMPs) are signalling proteins which interact with tissue structures in the body to enhance the skeletal development. Animals studies have shown that both BMP-2 and BMP-7 have the ability to increase the graft fixation strength in bone tunnels(36,37,38). A study by Sunder S et al in bovine models showed that demineralised bone matrix (DBM) is a source of BMPs which enhances tendon-bone healing and tendon-bone fixation strength(39). Further, Chen CH et al concluded that BMP-2 and periosteal progenitor cells induce rapid tendon-bone integration(21). However, there is much debate about the use of BMPs in ACLR surgeries in human being despite the theoretical advantages(21).
Discussion
The main goal of ACLR is to make the patient return to pre-injury level, and therefore return to sports, as soon as possible. In this direction, there have been a number of technological advances, in recent years, with respect to ACLR. Last few years have seen an increase in the number of publications on best surgical techniques, anatomical tunnel placements, use of scaffolds and augmentation with various biological products. Enhancing the tendon-to-bone healing has been the centre of research in ACLR(28,40,41).
It has been established that the tendon-bone healing occurs by collagen fibre scarring tissue which then reorganises to form a dense matrix. This is then followed by the appearance of Sharpey’s fibres. This collagen fiber continuity between the tendon and bone establishes the tendo-osseous junction and has been described as the earliest sign of osteo-integration(42,43).
Periosteum is rich in multipotent mesodermal cells and has osteogenic capacity. It has the ability to promote cartilage formation and also initiate enchondral ossification by inducing differentiation of mesenchymal cells into chondroblasts and subsequently into osteoblasts. It can also augment bone ingrowth into collagenous tissue and help induce ossification. When we incorporate periosteum in our graft by suturing on the surface of the tendon and then transplanting into the bony tunnel, the cambium layer of the periosteum serves as a fibrous layer between the tendon and bone interface. Studies have shown that by around 4 weeks, there is inter-digitation between the periosteum tissue and tendon resulting in progressive incorporation over time. Because of the effect of periosteum on promoting bony ingrowth and increasing the strength of the fixation, enveloping the tendon with it may be an effective way to enhance graft incorporation. Tunnel widening following ACLR is significantly greater with hamstring tendon. This is attributed to the greater distance from the normal insertion site and biomechanical point of action of the ACL, creating a larger force moment during graft cycling leading to greater expansion of the tunnels (44)
Platelet-rich plasma is another biological product that is frequently used to enhance tendon bone healing in ACLR. A prospective study by Radice et al compared the MRI findings between ACLR with biological augmentation and without biological augmentation(45). Post-operative MRIs showed that a 48% of time shortening, in healing, was achieved in augmentation group. Another study by Magnussen et al (46) compared 50 patients of allograft ACLR supplemented with platelet rich plasma, intra-operatively, with 50 patients of allograft ACLR without the use of platelet rich plasma using similar operative techniques. The results showed minor short-term clinical benefits, with biological augmentation, at two year post surgery. This, perhaps, indicates that biological augmentation is not that promising when allografts are used for ACLR. Further, a study by Mirzatolooei et al (47) evaluated tunnel diameters, in augmentation group and non-augmentation group, using CT scans on the day of surgery and at three months after surgery. Their results did not show a statistically significant change in the tunnel diameter between the augmentation group and non-augmentation group.
Conclusions
It appears, from the available literature, that biological augmentation in ACLR is an attractive option. However, at the moment, there is no concrete evidence to suggest that biologics work well with allografts. Besides, the tunnel widening issue still remains a major concern in ACLR and, there is conflicting evidence to support the idea of using biologics to address tunnel widening in ACLR. Although the jury is still out on specific advantages, it appears that there is no harm in using biologics in ACLR. It remains to be seen whether future level I studies will throw more light into the use of biological augmentation in ACLR procedures.
References
1. Kaplan N, Wickiewicz T, Warren R. Primary surgical treatment of anterior cruciate ligament ruptures: A long-term follow-up study. The American Journal of Sports Medicine. 1990;18(4):354-358.
2. Strand T, Molster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15 – 23 years postoperatively. Arch Orthop Trauma Surg. 2004;125(4):217-221.
3. Spindler K, Huston L, Wright R, Kaeding C, Marx R, Amendola A et al. The Prognosis and Predictors of Sports Function and Activity at Minimum 6 Years After Anterior Cruciate Ligament Reconstruction: A Population Cohort Study. The American Journal of Sports Medicine. 2010;39(2):348-359.
4. Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000 Oct;82-A(10):1387-97.
5. Visconti C, Kavalkovich K, Wu J, Niyibizi C. Biochemical Analysis of Collagens at the Ligament–Bone Interface Reveals Presence of Cartilage-Specific Collagens. Archives of Biochemistry and Biophysics. 1996;328(1):135-142.
6. Niyibizi C, Sagarrigo Visconti C, Gibson G, Kavalkovich K. Identification and immunolocalization of type X collagen at the ligament-bone interface. Biochem Biophys Res Commun. 1996;222:584-589.
7. Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S. Early anchoring collagen fibers at the bone—tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. Journal of Orthopaedic Research. 2001;19(5):873-880.
8. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993 Dec;75(12):1795-803.
9. Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344e351.
10. Lui P, Zhang P, Chan K, Qin L. Biology and augmentation of tendon-bone insertion repair. Journal of Orthopaedic Surgery and Research. 2010;5(1):59.
11. Ekdahl M, Wang J, Ronga M, Fu F. Graft healing in anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2008;16(10):935-947.
12. Goradia VK, Rochat MC, Grana WA, Rohrer MD, Prasad HS. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000 Summer;13(3):143-51.
13. Eriksson E. Vascular ingrowth into ACL-grafts. Knee Surgery, Sports Traumatology, Arthroscopy. 2008;16(4):341-341.
14. Chen C, Lee C. Biological fixation in anterior cruciate ligament surgery. Asia-Pacific Journal of Sports Medicine, Arthroscopy, Rehabilitation and Technology. 2014;1(2):48-53.
15. Gulotta L, Rodeo S. Biology of Autograft and Allograft Healing in Anterior Cruciate Ligament Reconstruction. Clinics in Sports Medicine. 2007;26(4):509-524.
16. Nebelung W, Becker R, Urbach D, Röpke M, Roessner A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003 May;123(4):158-63.
17. Weiler A, Hoffmann R, Bail H, Rehm O, Südkamp N. Tendon healing in a bone tunnel. Part II. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2002;18(2):124-135.
18. Wen C, Qin L, Lee K, Wan-Nar Wong M, Chan K. Influence of bone adaptation on tendon-to-bone healing in bone tunnel after anterior cruciate ligament reconstruction in a rabbit model. Journal of Orthopaedic Research. 2009;27(11):1447-1456.
19. Hays P. The Role of Macrophages in Early Healing of a Tendon Graft in a Bone Tunnel. The Journal of Bone and Joint Surgery (American). 2008;90(3):565.
20. Duthon V, Barea C, Abrassart S, Fasel J, Fritschy D. Anatomy of the anterior cruciate ligament. Knee Surgery, Sports Traumatology, Arthroscopy. 2006;14(3):204–213.
21. Chen C, Liu H, Tsai C, Yu C, Lin I, Hsiue G. Photoencapsulation of Bone Morphogenetic Protein-2 and Periosteal Progenitor Cells Improve Tendon Graft Healing in a Bone Tunnel. The American Journal of Sports Medicine. 2007;36(3):461-473.
22. Midwood K, Williams L, Schwarzbauer J. Tissue repair and the dynamics of the extracellular matrix. The International Journal of Biochemistry & Cell Biology. 2004;36(6):1031-1037.
23. Zhang J, Wang J. Platelet-Rich Plasma Releasate Promotes Differentiation of Tendon Stem Cells Into Active Tenocytes. The American Journal of Sports Medicine. 2010;38(12):2477-2486.
24. Bielecki T, Gazdzik T, Arendt J, Szczepanski T, Krol W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: AN IN VITRO STUDY. Journal of Bone and Joint Surgery – British Volume. 2007;89-B(3):417-420.
25. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012 Jun;13(7):1185-95.
26. Filardo G, Kon E, Roffi A, Di Matteo B, Merli M, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surgery, Sports Traumatology, Arthroscopy. 2013;23(9):2459-2474.
27. Baksh N, Hannon C, Murawski C, Smyth N, Kennedy J. Platelet-Rich Plasma in Tendon Models: A Systematic Review of Basic Science Literature. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2013;29(3):596-607.
28. Boswell S, Cole B, Sundman E, Karas V, Fortier L. Platelet-Rich Plasma: A Milieu of Bioactive Factors. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2012;28(3):429-439.
29. Ritsila VA, Santavirta S, Alhopuro S, Poussa M, Jaroma H, Rubak JM, et al. Periosteal and perichondral grafting in reconstructive surgery. Clin Orthop Relat Res 1994;302:259‑65
30. Rubak J. Osteochondrogenesis of Free Periosteal Grafts in the Rabbit Iliac Crest. Acta Orthopaedica Scandinavica. 1983;54(6):826-831.
31. Chen C, Chen W, Shih C, Yang C, Liu S, Lin P. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2003;19(3):290-296.
32. Youn I, Jones D, Andrews P, Cook M, Suh J. Periosteal Augmentation of a Tendon Graft Improves Tendon Healing in the Bone Tunnel. Clinical Orthopaedics and Related Research. 2004;419:223-231.
33. Chen C, Chen W, Shih C. Enveloping of Periosteum on the Hamstring Tendon Graft in Anterior Cruciate Ligament Reconstruction (SS-69). Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2002;18(5):53-54.
34. Chen C, Chen W, Shih C, Chou S. Arthroscopic anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft. Knee Surgery, Sports Traumatology, Arthroscopy. 2004;12(5).
35. Chen C, Chang C, Su C, Wang K, Liu H, Yu C et al. Arthroscopic Single-Bundle Anterior Cruciate Ligament Reconstruction With Periosteum-Enveloping Hamstring Tendon Graft: Clinical Outcome at 2 to 7 Years. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(7):907-917.
36. Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J.Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002 Jul;84-A(7):1123-31.
37. Axelrad T, Einhorn T. Bone morphogenetic proteins in orthopaedic surgery. Cytokine & Growth Factor Reviews. 2009;20(5-6):481-488.
38. Mihelic R. Bone Morphogenetic Protein-7 (Osteogenic Protein-1) Promotes Tendon Graft Integration in Anterior Cruciate Ligament Reconstruction in Sheep. American Journal of Sports Medicine. 2004;32(7):1619-1625.
39. Sundar S, Pendegrass C, Blunn G. Tendon bone healing can be enhanced by demineralized bone matrix: A functional and histological study. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;88B(1):115-122.
40. Gulotta L, Wiznia D, Cunningham M, Fortier L, Maher S, Rodeo S. What’s New in Orthopaedic Research. The Journal of Bone and Joint Surgery (American). 2011;93(22).
41. Chang CH, Chen CH, Liu HW, Whu SW, Chen SH, Tsai CL, Hsiue GH. Bioengineered periosteal progenitor cell sheets to enhance tendon-bone healing in a bone tunnel. Biomed J. 2012 Nov-Dec;35(6):473-80.
42. Liu S, Panossian V, Al-Shaikh R, Tomin E, Shepherd E, Finerman G et al. Morphology and Matrix Composition During Early Tendon to Bone Healing. Clinical Orthopaedics and Related Research. 1997;339:253-260.
43. Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone–Patellar tendon–Bone graft in anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2001;17(5):461-476.
44. Fahey M, Indelicato P. Bone Tunnel Enlargement After Anterior Cruciate Ligament Replacement. The American Journal of Sports Medicine. 1994;22(3):410-414.
45. Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of Magnetic Resonance Imaging Findings in Anterior Cruciate Ligament Grafts With and Without Autologous Platelet-Derived Growth Factors. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(1):50-57.
46. Magnussen R, Flanigan D, Pedroza A, Heinlein K, Kaeding C. Platelet rich plasma use in allograft ACL reconstructions: Two-year clinical results of a MOON cohort study. The Knee. 2013;20(4):277-280.
47. Mirzatolooei F, Alamdari M, Khalkhali H. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: A randomised clinical trial. The Bone & Joint Journal. 2013;95-B(1):65-69
| How to Cite this article:. Shetty VD, Alva K, Gupta V. Biologics in Primary Anterior Cruciate Ligament Reconstruction. Asian Journal of Arthroscopy Apr- June 2016;1(1):25-28 . |
 Dr Vijay D Shetty |
 Dr Karan Alva |
 Dr Varun Gupta |